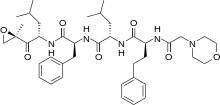
Carfilzomib
synthesis………http://newdrugapprovals.org/2014/08/05/amgens-multiple-myeloma-drug-shows-promise-in-phase-3-trial/
supplemental New Drug Application filed
Amgen and its subsidiary Onyx Pharmaceuticals have submitted filings for their multiple myeloma drug Kyprolis (carfilzomib) on both sides of the Atlantic.
The companies are seeking approval to market their drug for the treatment of patents with relapsed multiple myeloma who have received at least one prior therapy.
read all at…………http://www.pharmatimes.com/Article/15-01-28/Amgen_Onyx_file_multiple_myeloma_drug_in_US_EU.aspx
synthesis………http://newdrugapprovals.org/2014/08/05/amgens-multiple-myeloma-drug-shows-promise-in-phase-3-trial/
 |
|
| Systematic (IUPAC) name | |
|---|---|
| (S)-4-Methyl-N-((S)-1-(((S)-4-methyl-1-((R)-2-methyloxiran-2-yl)-1-oxopentan-2-yl)amino)-1-oxo-3-phenylpropan-2-yl)-2-((S)-2-(2-morpholinoacetamido)-4-phenylbutanamido)pentanamide | |
| Clinical data | |
| Trade names | Kyprolis |
| Licence data | US FDA:link |
|
|
| Legal status | |
| Routes | Intravenous |
| Identifiers | |
| CAS number | 868540-17-4 |
| ATC code | L01XX45 |
| PubChem | CID 11556711 |
| ChemSpider | 9731489 |
| KEGG | D08880 |
| ChEMBL | CHEMBL451887 |
| Synonyms | PX-171-007 |
| Chemical data | |
| Formula | C40H57N5O7 |
| Molecular mass | 719.91 g mol |
Filed under: sNDA Tagged: amgen, Carfilzomib, multiple myeloma, Onyx Pharmaceuticals, supplemental New Drug Application