AZASETRON, NAZASETRON
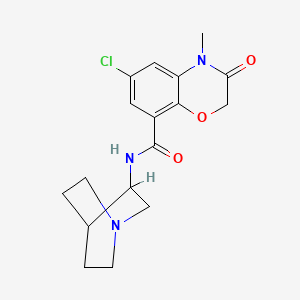
N-(1-azabicyclo[2.2.2]octan-8-yl)-6-chloro-4-methyl-3-oxo-1,4-benzoxazine-8-carboxamide
6-chloro-3,4-dihydro-4-methyl-3-oxo-N-(3-quinuclidinyl)-2H-1,4-benzoxazine-8-carboxamide
123039-99-6 , 123040-69-7 (free base)
141922-90-9 HYD SALT
Y-25130 (hydrochloride)LAUNCHED 1994 AS HCL SALT FORM SEROTONE
a selective 5-HT3 receptor antagonist , ANTIEMETIC
- UNII-77HC7URR9Z
Mitsubishi Tanabe Pharma, JAPAN TOBACCO (Originator)
FOR..Nausea and Vomiting, Treatment of
Prokinetic Agents

Azasetron is an antiemetic which acts as a 5-HT3 receptor antagonist.
Chemical and Pharmaceutical Bulletin, 1992 , vol. 40, 3 p. 624 – 630, ENTRY 15 , MP 305 OF HCL SALT
Biological and Pharmaceutical Bulletin, 2006 , vol. 29, 9 p. 1931 – 1935, AS MERCK 903
US 4892872…
CN 101786963…
WO 1996001630..
WO 2006006595..
WO 2007134077..
CN 102526740, CN 102451166, US 4892872, JP 2008297277
US5773436 A1, WO2006/119295 A2, EP1336602 A1, US4892872 A1,
US2003/18008 A1, US2002/147197 A1, US4892872


The nitration of 5-chloro-2-hydroxybenzoic acid methyl ester (I) with nitric acid in sulfuric acid gives 5-chloro-2-hydroxy-3-nitrobenzoic acid methyl ester (II), which is reduced with Fe and NH4Cl in water yielding 3-amino-5-chloro-2-hydroxybenzoic acid methyl ester (III). The cyclization of (III) with chloroacetyl chloride (IV) by means of NaHCO3 in CHCl3 – water affords 6-chloro-3-oxo-3,4-dihydro-2H-1,4-benzoxazine-8-carboxylic acid methyl ester (V), which is methylated with methyl iodide and K2CO3 in DMF affording the corresponding 4-methyl derivative (VI). Hydrolysis of (VI) with ethanolic NaOH gives the corresponding acid (VII), which by treatment with refluxing SOCl2 is converted into its acyl chloride (VIII). Finally, this compound is condensed with 3-aminoquinuclidine (IX) by means of N-methylmorpholine (NMM) in CHCl3.
………………
SYNTHESIS
Azasetron hydrochloride (Azasetron hydrochloride) is a secondary cancer drugs, mainly synthetic route is as follows
[0003]

[0004] In EP 0313393; JP 1989207290; JP 1990005415; US 4892872; Eur.Pat.Appl., 313393,, Chem pharm Bull, 1992,40 (3) :624-630 reported the synthesis of intermediates III is with ammonium chloride, iron as the reducing agent, the nitro substrate was dissolved in toluene and added dropwise to a reduction of the media inside, the study found that this approach has a number of disadvantages, notably the reaction can not be completely, while post-processing is very troublesome, purity of the product obtained is poor, and difficult purification. Although the “Chinese Medicinal Chemistry” magazine, 10 (2) ,138-140; 2000, the author hydrochloride instead of ammonium chloride, but still need the nitro group was dissolved in toluene was added dropwise, in fact, the nitro compound in toluene the solubility is limited, and in the process will precipitate dropwise addition, there are also incomplete reaction, impurities, and complicated post-treatment problems.
[0005] In the “China Pharmaceutical Industry” magazine 34 (3) ,214-215; 2003 in order to restore hydrosulfite as reductant,
Azasetron preparation of intermediates, the steps are as follows:
[0015] (I) A mixture of 3 – nitro-5 – chloro-salicylate added glacial acetic acid was stirred for 30-40 minutes, add water, temperature 30 ~ 100 ° C, adding iron powder, 2.5-3 hour plus finished, the addition was completed, at a temperature of 30 ~ 100 ° C the reaction for 5 to 10 hours; give 3 – amino-5-chloro-salicylate mixture;
[0016] The mass ratio of the added material is 3 – nitro-5 – chloro-salicylate: acetic acid: water: iron = 1: 5 ~ 10: 5 ~ 10: 0.5 ~ I;
[0017] (2) obtained in the step ⑴ added to a mixture of glacial acetic acid, glacial acetic acid added in the amount of step ⑴ same amount of glacial acetic acid, 70 ° C was stirred for 30-35 minutes, filtered through celite to remove the iron sludge to obtain filter cake; this procedure to that step (I) the resulting product was dissolved in glacial acetic acid solvent sufficient, in order to facilitate post-processing; while removing impurities.
[0018] (3) The filter cake was washed with an organic solvent, the combined filtrate and stirred for 10-15 minutes, allowed to stand for 30-35 minutes, the organic layer was separated; aqueous phase was extracted once again with an organic solvent, the combined organic solution was washed three times , mention made three – amino -5 chloro methyl salicylate (intermediate III) sulphate solution.
[0019] (4) of step (3) of 3 – amino-5-chloro-salicylate was added a saturated NaHCO3 solution, saturated NaHCO3 solution and the amount of acetic acid in step ⑴ same volume, cooling to _1 ° C ~ 0 ° C, control the temperature -1 ° C_5 ° C was added dropwise acetyl chloride, drop Bi. Reaction 2-3 hours. To give 3 – (2 – chloro-acetylamino)-5-chloro-mixed solution of methyl salicylate;
[0020] The amount of chloroacetyl chloride is added in step ⑴ source material 3 – nitro-5 – chloro molar ratio of methyl salicylate
I: 0.9-0.95.
[0021] (5) obtained in the step ⑷ 3 – (2 – chloro-acetylamino) methyl salicylate _5 chloride mixture was heated to 30-40 ° C, stirred for 1-1.5 hours; organic layer was separated, water phase extracted once again with an organic solvent, the combined organic layer was washed with water three times, separated and the organic layer is directly subjected to atmospheric distillation, the organic solvent is distilled off, distillation was complete, methanol or ethanol as the crystallization solvent, 80 ° C under stirring for 2.5 hours under reflux , to give 3 – (2 – chloro-acetylamino) _5 chlorine in alcohol solution of methyl salicylate. This step is intended to 3 – (2 – chloro-acetylamino)-5-chloro methyl salicylate purification.
[0022] (6) in the step (5) cooling to room temperature, an alcoholic solution, crystallization, centrifugation, and washed with ethanol crystal; 80 ° C drying in needle-like crystals 3 – (2 – chloro-acetylamino) _5 Chlorine methyl salicylate (intermediate IV).
……………………………………………………
USEFUL PATENTS
|
6-31-1998
|
Use of serotonin antagonists for treating fibromyalgia
|
|
|
11-28-1997
|
COMPOSITIONS COMPRISING CONJUGATES OF CIS-DOCOSAHEXAENOIC ACID AND TAXOTERE
|
|
|
11-28-1997
|
DHA-PHARMACEUTICAL AGENT CONJUGATES DHA-PHARMACEUTICAL AGENT CONJUGATES
|
|
|
11-28-1997
|
CONJUGATES OF CIS-DOCOSAHEXAENOIC ACID AND PACLITAXEL
|
|
|
3-28-1997
|
5-HT3 RECEPTOR ANTAGONISTS FOR DYSKINESIA
|
|
|
2-19-1997
|
Nasally administrable compositions
|
|
|
1-26-1996
|
PREVENTIVE OR REMEDY FOR IRRITABLE BOWEL SYNDROME OR DIARRHEA
|
|
|
3-17-1994
|
Benzoxazine compounds and pharmaceutical use thereof.
|
|
|
1-10-1990
|
Benzoxazine compounds and pharmaceutical use thereof
|
|
12-17-1999
|
MULTIVALENT AGONISTS, PARTIAL AGONISTS, INVERSE AGONISTS AND ANTAGONISTS OF THE 5-HT3 RECEPTORS MULTIVALENT AGONISTS, PARTIAL AGONISTS, INVERSE AGONISTS AND ANTAGONISTS OF THE 5-HT>3< RECEPTORS MULTIVALENT AGONISTS, PARTIAL AGONISTS, INVERSE AGONISTS AND ANTAGONISTS OF THE 5-HT3 RECEPTORS
|
|
|
11-17-1999
|
Use of serotonin antagonists for treating fibromyalgia
|
|
|
11-5-1999
|
CNRE BINDING FACTORS AND USES THEREOF
|
|
|
7-23-1999
|
TRANSGLUTAMINASE LINKAGE OF AGENTS TO TISSUE TRANSGLUTAMINASE LINKAGE OF AGENTS TO TISSUE
|
|
|
7-7-1999
|
Taxane compounds and compositions
|
|
|
6-25-1999
|
ORAL DELIVERY FORMULATION
|
|
|
4-16-1999
|
MEDICAMENTS MEDICAMENTS
|
|
|
2-5-1999
|
CHEMOTHERAPY SYNERGISTIC AGENT
|
|
|
10-30-1998
|
USE OF 5HT3 ANTAGONISTS FOR PROMOTING INTESTINAL LAVAGE
|
|
|
8-19-1998
|
DHA-pharmaceutical agent conjugates of taxanes
|
|
8-7-2009
|
ALISKIREN MODULATION OF NEUROGENESIS
|
|
|
7-3-2003
|
USE OF 5HT3 ANTAGONISTS FOR PROMOTING INTESTINAL LAVAGE
|
|
|
9-18-2002
|
Inhibition of emetic effect of metformin with 5-HT3 receptor antagonists
|
|
|
11-8-2001
|
CONJUGATES OF CIS-DOCOSAHEXAENOIC ACID AND PACLITAXEL
|
|
|
6-14-2001
|
Nasally administrable compositions
|
|
|
12-22-2000
|
RECEPTOR AGONISTS AND ANTAGONISTS COMPOUND FOR USE AS A MEDICAMENT FOR TREATMENT OF DISORDERS INVOLVING BRONCHOCONTRACTION COMPOUND FOR USE AS A MEDICAMENT FOR TREATMENT OF DISORDERS INVOLVING BRONCHOCONTRACTION
|
|
|
12-15-2000
|
PHARMACEUTICAL COMPOSITION FOR INTRANASAL USE OF ACTIVE SUBSTANCES THAT ARE INSOLUBLE AND/OR HARDLY SOLUBLE IN WATER
|
|
|
11-31-2000
|
ANTI-TUMOR COMPRISING BOROPROLINE COMPOUNDS
|
|
|
11-30-2000
|
TRANSGLUTAMINASE LINKAGE OF AGENTS TO TISSUE
|
|
11-17-2000
|
FATTY ACID-N-SUBSTITUTED INDOL-3-GLYOXYL-AMIDE COMPOSITIONS AND USES THEREOF
|
|
|
10-12-2000
|
COMPOSITIONS COMPRISING CONJUGATES OF CIS-DOCOSAHEXAENOIC ACID AND TAXOTERE
|
|
|
9-15-2000
|
FATTY ACID-ANTICANCER CONJUGATES AND USES THEREOF FATTY ACID-ANTICANCER CONJUGATES AND USES THEREOF
|
|
|
7-20-2000
|
$g(b)2-ADRENERGIC RECEPTOR AGONISTS $g(b)2-ADRENERGIC RECEPTOR AGONISTS
|
|
|
7-7-2000
|
USE OF CD40 ENGAGEMENT TO ALTER T CELL RECEPTOR USAGE USE OF CD40 ENGAGEMENT TO ALTER T CELL RECEPTOR USAGE USE OF CD40 ENGAGEMENT TO ALTER T CELL RECEPTOR USAGE
|
|
|
6-28-2000
|
Taxanes
|
|
|
5-32-2000
|
$g(b)2-ADRENERGIC RECEPTOR AGONISTS
|
|
|
1-14-2000
|
NOVEL INDOLOCARBAZOLE DERIVATIVES USEFUL FOR THE TREATMENT OF NEURODEGENERATIVE DISEASES AND CANCER
|
|
|
1-12-2000
|
Indolocarbazole derivatives useful for the treatment of neurodegenerative diseases and cancer
|
|
|
12-30-1999
|
METHODS FOR IDENTIFYING NOVEL MULTIMERIC AGENTS THAT MODULATE RECEPTORS METHODS FOR IDENTIFYING NOVEL MULTIMERIC AGENTS THAT MODULATE RECEPTORS
|
………..AZASETRON
AZASETRON
NMR OF HCL SALT
http://file.selleckchem.com/downloads/nmr/S210601-Azasetron-hydrochloride-NMR-Selleck.pdf
…………….
SYNTHESIS
EXAMPLE 15
To a solution of 3.0 g of 3-aminoquinuclidine and 3.0 g of N-methylmorpholine in 60 ml of chloroform is added 6.2 g of 6-chloro-3,4-dihydro-4-methyl-3-oxo-2H-1,4-benzoxazine-8-carboxylic acid chloride under cooling and stirring followed by stirring for 2 hours. The resultant solution is washed with water, aqueous sodium hydrogen carbonate and then water, and dried over magnesium sulfate. After the solvent is distilled off under reduced pressure, the residue is recrystallized from ethanol-isopropyl ether and treated with ethanolic hydrochloric acid to give 6-chloro-3,4-dihydro-4-methyl-3-oxo-N-(3-quinuclidinyl)-2H-1,4-benzoxazine-8-carboxamide hydrochloride, melting at 281
………..
SYNTHESIS AND + AND – ISOMERS
EXAMPLE 36
A solution of 6 g of 6-chloro-3,4-dihydro-4-methyl-3-oxo-N-(3-quinuclidinyl)-2H-1,4-benzoxazine-8-carboxamide and 2.7 g of D-(-)-tartaric acid in 100 ml of methanol and 200 ml of ethanol is allowed to stand. The precipitated crystals are collected by filtration and recrystallized from methanol repeatedly to give the R-(+) isomer, [α].sub.D.sup.25 =+36.72 (c=1, chloroform), melting at 177 with ethanolic hydrochloric acid, and then the precipitated crystals are collected by filtration and dried to give the R-(+) isomer hydrochloride, [α].sub.D.sup.25 =+1.4 (c=1, water), melting at 309
By using L-(+)-tartaric acid in a similar manner, the R-(-) optical isomer, [α].sub.D.sup.25.5 =-36.76 (c=1, chloroform), melting at 176 [α].sub.D.sup.25.5 =-1.2 (c=1, water), melts at 310

THANKS AND REGARD’S
DR ANTHONY MELVIN CRASTO Ph.D
GLENMARK SCIENTIST , NAVIMUMBAI, INDIA
did you feel happy, a head to toe paralysed man’s soul in action for you round the clock
need help, email or call me
I was paralysed in dec2007, Posts dedicated to my family, my organisation Glenmark, Your readership keeps me going and brings smiles to my family
Filed under: Uncategorized Tagged: AZASETRON, NAZASETRON
