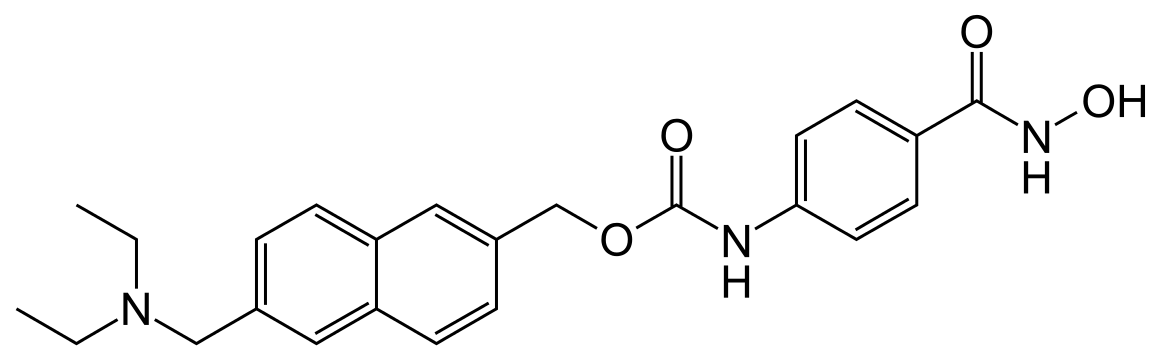
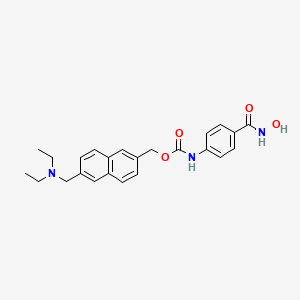

Givinostat (INN[1]) or gavinostat (originally ITF2357) is a histone deacetylase inhibitor with potential anti-inflammatory, anti-angiogenic, and antineoplastic activities.[2] It is a hydroxamate used in the form of its hydrochloride.
Givinostat is in numerous phase II clinical trials (including for relapsed leukemias and myelomas),[3] and has been granted orphan drug designation in the European Union for the treatment of systemic juvenile idiopathic arthritis[4] and polycythaemia vera.[5]
In 2010, orphan drug designation was assigned in the E.U. for the treatment of systemic-onset juvenile idiopathic arthritis and for the treatment of polycythemia vera. In 2013, this designation was assigned by the FDA for the treatment of Duchenne’s muscular dystrophy and for the treatment of Becker’s muscular dystrophy.
ITF2357 was discovered at Italfarmaco of Milan, Italy. It was patented in 1997 and first described in the scientific literature in 2005.[6][7]
Givinostat hydrochloride, an orally active, synthetic inhibitor of histone deacetylase, is being evaluated in several early clinical studies at Italfarmaco, including studies for the treatment of myeloproliferative diseases, polycythemia vera, Duchenne’s muscular dystrophy and periodic fever syndrome. The company was also conducting clinical trials for the treatment of Crohn’s disease and chronic lymphocytic leukemia; however, the trials were terminated.
No recent development has been reported for research into the treatment of juvenile rheumatoid arthritis, for the treatment of multiple myeloma and for the treatment of Hodgkin’s lymphoma.
Muscular dystrophies (MDs) include a heterogeneous group of genetic diseases invariably leading to muscle degeneration and impaired function. Mutation of nearly 30 genes gives rise to various forms of muscular dystrophy, which differ in age of onset, severity, and muscle groups affected (Dalkilic I, Kunkel LM. (2003) Muscular dystrophies: genes to pathogenesis. Curr. Opin. Genet. Dev. 13:231-238). The most common MD is the Duchenne muscular dystrophy (DMD), a severe recessive X-linked disease which affects one in 3,500 males, characterized by rapid progression of muscle degeneration, eventually leading to loss of ambulation and death within the second decade of life.
Attempts to replace or correct the mutated gene, by means of gene or cell therapy, might result in a definitive solution for muscular dystrophy, but this is not easy to achieve. Alternative strategies that prevent or delay muscle degeneration, reduce inflammation or promote muscle metabolism or regeneration might all benefit patients and, in the. future, synergize with gene or cell therapy. Steroids that reduce inflammation are currently the only therapeutic tool used in the majority of DMD patients (Cossu G, Sampaolesi M . (2007) New therapies for Duchenne muscular dystrophy: challenges, prospects and clinical trials. TRENDS Mol . Med. 13:520-526).
Diethyl- [ 6- ( 4-hydroxycarbamoyl-phenyl-carbamoyloxy- methyl ) -naphthalen-2-yl-methyl ] -ammonium chloride , which is described in WO 97/43251 (anhydrous form) and in WO 2004/065355 (monohydrate crystal form), herein both incorporated by reference, is an anti-inflammatory agent which is able to inhibit the synthesis of the majority of pro-inflammatory cytokines whilst sparing anti-inflammatory ones. Diethyl- [ 6- ( 4-hydroxycarbamoyl-phenyl-carbamoyloxy- methyl ) -naphthalen-2-yl-methyl ] -ammonium chloride is also known as ITF2357.
The monohydrate crystal form of diethyl- [ 6- ( 4- hydroxycarbamoyl-phenyl-carbamoyloxy-methy1 ) – naphthalen-2-yl-methyl ] -ammonium chloride is known as Givinostat .
Givinostat is being evaluated in several clinical studies, including studies for the treatment of myeloproliferative diseases, polycythemia vera, periodic fever syndrome, Crohn’s disease and systemic- onset juvenile idiopathic arthritis. Orphan drug designation was assigned in the E.U. for the treatment of systemic-onset juvenile idiopathic arthritis and for the treatment of polycythemia vera.
Givinostat has been recently found to act also as a Histone Deacetylase inhibitor (WO 2011/048514).
Histone deacetylases ( HDAC ) are a family of enzymes capable of removing the acetyl group bound to the lysine residues in the N-terminal portion of histones or in other proteins.
HDACs can be subdivided into four classes, on the basis of structural homologies. Class I HDACs (HDAC 1, 2, 3 and 8) are similar to the RPD3 yeast protein and are located in the cell nucleus. Class II HDACs (HDAC 4, 5, 6, 7, 9 and 10) are similar to the HDA1 yeast protein and are located both in the nucleus and in the cytoplasm. Class III HDACs are a structurally distinct form of NAD-dependent enzymes correlated with the SIR2 yeast protein. Class IV (HDAC 11) consists at the moment of a single enzyme having particular structural characteristics. The HDACs of classes I, II and IV are zinc enzymes and can be inhibited by various classes of molecule: hydroxamic acid derivatives, cyclic tetrapeptides , short-chain fatty acids, aminobenzamides , derivatives of electrophilic ketones, and the like. Class III HDACs are not inhibited by hydroxamic acids, and their inhibitors have structural characteristics different from those of the other classes .
The expression “histone deacetylase inhibitor” in relation to the present invention is to be understood as meaning any molecule of natural, recombinant or synthetic origin capable of inhibiting the activity of at least one of the enzymes classified as histone deacetylases of class I, class II or class IV.
Although HDAC inhibitors, as a class, are considered to be potentially useful as anti-tumor agents, it is worth to note that, till now, only two of them (Vorinostat and Romidepsin) have been approved as drugs for the cure of a single tumor form (Cutaneous T-cell lymphoma ) .
It is evident that the pharmaceutical properties of each HDAC inhibitor may be different and depend on the specific profile of inhibitory potency, relative to the diverse iso-enzymes as well as on the particular pharmacokinetic behaviour and tissue distribution.
Some HDAC inhibitors have been claimed to be potentially useful, in combination with other agents, for the treatment of DMD (WO 2003/033678, WO 2004/050076, Consalvi S. et al. Histone Deacetylase Inhibitors in the Treatment of Muscular Dystrophies: Epigenetic Drugs for Genetic Diseases. (2011) Mol. Med. 17 : 457-465 ) .
The potential therapeutic use of HDAC inhibitors in DMD may however be hampered by the possible harmful effects of these relatively toxic agents, especially when used for long-term therapies in paediatric patients .
Givinostat, as anti-inflammatory agent, has been already used in a phase II study in children with Systemic Onset Juvenile Idiopathic Arthritis; Givinostat administered at 1.5 mg/kg/day for twelve weeks achieved ACR Pedi 30, 50 and 70 improvement of approximately 70% (Vojinovic J, Nemanja D. (2011) HDAC Inhibition in Rheumatoid Arthritis and Juvenile Idiopathic Arthritis. Mol. Med 17:397-403) showing only a limited number of mild or moderate but short lasting, adverse effects.
To date more than 500 patients (including 29 children) have been treated with Givinostat. Repeated dose toxicity studies were carried out in dogs, rats and monkeys. Oral daily doses of the drug were administered up to nine consecutive months. The drug was well tolerated with no overt toxicity at high doses. The “no adverse effect levels” (NOAEL) ranged from 10 to 25 mg/kg/day depending on the animal species and the duration of treatment.
In juvenile animals Givinostat at 60 mg/kg/day did not affect the behavioural and physical development and reproductive performance of pups.
No genotoxic effect was detected for Givinostat in the mouse lymphoma assay and the chromosomal aberration assay in vitro and in the micronucleus test and UDS test in vivo.
| Patent | Submitted | Granted |
|---|---|---|
| Monohydrate hydrochloride of the 4-hydroxycarbamoyl-phenyl)-carbamic acid (6-diethylaminomethyl-naphtalen-2-yl) ester [US7329689] | 2005-11-03 | 2008-02-12 |
Adverse effects
In clinical trials of givinostat as a salvage therapy for advanced Hodgkin’s lymphoma, the most common adverse reactions were fatigue (seen in 50% of participants), mild diarrhea or abdominal pain (40% of participants), moderate thrombocytopenia (decreased platelet counts, seen in one third of patients), and mild leukopenia (a decrease in white blood cell levels, seen in 30% of patients). One-fifth of patients experienced prolongation of the QT interval, a measure of electrical conduction in the heart, severe enough to warrant temporary suspension of treatment.[8]
Mechanism of action
Givinostat inhibits class I and class II histone deacetylases (HDACs) and several pro-inflammatory cytokines. This reduces expression of tumour necrosis factor (TNF), interleukin 1α and β, and interleukin 6.[7]
It also has activity against cells expressing JAK2(V617F), a mutated form of the janus kinase 2 (JAK2) enzyme that is implicated in the pathophysiology of many myeloproliferative diseases, including polycythaemia vera.[9][10] In patients with polycythaemia, the reduction of mutant JAK2 concentrations by givinostat is believed to slow down the abnormal growth of erythrocytes and ameliorate the symptoms of the disease.[5]
………………….

PATENT
https://www.google.com/patents/WO2004065355A1?cl=en
Hydrochloride of (6-diethylaminomethyl-naphthalen-2-yl)- methyl ester of (4-hydroxycarbamoylphenyl)-carbamic acid (II)
has been described in US patent 6,034,096 as a derivative of hydroxamic acid having anti-inflammatory and immunosuppressive activity, probably owing to the ability thereof to inhibit the production of pro-inflammatory cyto ines. This compound is obtained according to
Example 12 of the above-mentioned patent as an anhydrous, amorphous, hygroscopic, deliquescent solid which is difficult to handle.
crystalline form of monohydrous hydrochloride of
(6-diethylaminomethyl-naphthalen-2-yl)-methyl ester of
(4~hydroxycarbamoylphenyl)-carbamic acid (I).
This form is particularly advantageous from the industrial perspective because it is stable and simpler to handle than the anhydrous and amorphous form described above.
………………

PATENT
http://www.google.co.in/patents/US7329689
Hydrochloride of (6-diethylaminomethyl-naphthalen-2-yl)-methyl ester of (4-hydroxycarbamoylphenyl)-carbamic acid (II)
has been described in U.S. Pat. No. 6,034,096 as a derivative of hydroxamic acid having anti-inflammatory and immunosuppressive activity, probably owing to the ability thereof to inhibit the production of pro-inflammatory cytokines. This compound is obtained according to Example 12 of the above-mentioned patent as an anhydrous, amorphous, hygroscopic, deliquescent solid which is difficult to handle.
The 4-(6-diethylaminomethyl-naphthalen-2-ylmethoxycarbonylamino)-benzoic acid can be prepared as described in Example 12, point C, of U.S. Pat. No. 6,034,096.
The acid (1.22 kg, 3 moles) was suspended in THF (19 l) and the mixture was agitated under nitrogen over night at ambient temperature. The mixture was then cooled to 0° C. and thionyl chloride (0.657 l, 9 moles) was added slowly, still under nitrogen, with the temperature being maintained below 10° C. The reaction mixture was heated under reflux for 60 minutes, DMF (26 ml) was added and the mixture was further heated under reflux for 60 minutes.
The solvent was evaporated under vacuum, toluene was added to the residue and was then evaporated. This operation was repeated twice, then the residue was suspended in THF (11.5 l) and the mixture was cooled to 0° C.
The mixture was then poured into a cold solution of hydroxylamine (50% aq., 1.6 l, 264 moles) in 5.7 l of water. The mixture was then cooled to ambient temperature and agitated for 30 minutes. 6M HCl was added until pH 2 was reached and the mixture was partially evaporated under vacuum in order to eliminate most of the THF. The solid was filtered, washed repeatedly with water and dissolved in a solution of sodium bicarbonate (2.5%, 12.2 l). The solution was extracted with 18.6 l of a mixture of THF and ethyl acetate (2:1 v/v). 37% HCl (130 ml) were added to the organic layer in order to precipitate the monohydrate of the (6-diethylaminomethyl-naphthalen-2-yl)-methyl ester hydrochloride of the (4-hydroxycarbamoyl-phenyl)-carbamic acid. If necessary, this operation can be repeated several times to remove any residues of the original acid.
Finally, the solid was dried under vacuum (approximately 30 mbar, 50° C.), producing 0.85 kg (60%) of compound (I).
HPLC purity: 99.5%; water content (Karl Fischer method): 3.8%; (argentometric) assay: 99.8%.
| Elemental analysis | |||||
| C % | H % | Cl % | N % | ||
| Calculated for | 60.56 | 6.35 | 7.45 | 8.83 | |
| C24H30ClN3O5 | |||||
| Found | 61.06 | 6.48 | 7.48 | 8.90 | |
…..
PATENT
http://www.google.co.in/patents/US20120302633
…………………..
http://www.google.com/patents/US6034096
EXAMPLE 12
4-[6-(Diethylaminomethyl)naphth-2-ylmethyloxycarbamoyl]-benzohydroxamic acid hydrochloride
A. 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI) (22.2 g, 115 mmol) was added to a solution of 2,6-naphthalenedicarboxylic acid (25 g, 115 mmol) and hydroxybenzotriazole (15.6 g, 115 mmol) in dimethylformamide (1800 ml) and the mixture was stirred at room temperature for 2 hours. Diethyl amine (34.3 ml, 345 mmol) was added and the solution was stirred overnight at room temperature. The solvent was then evaporated under reduced pressure and the crude was treated with 1N HCl (500 ml) and ethyl acetate (500 ml), insoluble compounds were filtered off and the phases were separated. The organic phase was extracted with 5% sodium carbonate (3×200 ml) and the combined aqueous solutions were acidified with concentrated HCl and extracted with ethyl acetate (3×200 ml). The organic solution was then washed with 1N HCl (6×100 ml), dried over anhydrous sodium sulphate and the solvent was removed under reduced pressure yielding 18.5 g (Yield 60%) of pure 6-(diethylaminocarbonyl)-2-naphthalenecarboxylic acid; m.p.=122-124° C.
1 H-NMR d 8.67 (s, 1H), 8.25-8.00 (m, 4H), 7.56 (d, 1H), 3.60-3.20 (m, 4H), 1.30-1.00 (m, 6H).
B. A solution of 6-(diethylaminocarbonyl)-2- naphthalenecarboxylic acid (18 g, 66 mmol) in THF (200 ml) was slowly added to a refluxing suspension of lithium aluminium hydride (7.5 g, 199 mmol) in THF (500 ml). The mixture was refluxed for an hour, then cooled at room temperature and treated with a mixture of THF (25 ml) and water (3.5 ml), with 20% sodium hydroxide (8.5 ml) and finally with water (33 ml). The white solid was filtered off and the solvent was removed under reduced pressure. Crude was dissolved in diethyl ether (200 ml) and extracted with 1N HCl (3×100 ml). The aqueous solution was treated with 32% sodium hydroxide and extracted with diethyl ether (3×100 ml). The organic solution was dried over anhydrous sodium sulphate and the solvent was removed under reduced pressure yielding 12.7 g (79% yield) of pure 6-(diethylaminomethyl)-2-naphthalenemethanol as thick oil.
1 H-NMR d 7.90-7.74 (m, 4H), 7.49 (m, 2H), 5.32 (t, 1H, exchange with D2 O), 4.68 (d, 2H), 3.69 (s, 2H), 2.52 (q, 4H), 1.01 (t, 6H).
C. A solution of 6-(diethylaminomethyl)-2-naphthalene-methanol (12.5 g, 51 mmol) and N,N’-disuccinimidyl carbonate (13.2 g, 51 mmol) in acetonitrile (250 ml) was stirred at room temperature for 3 hours, then the solvent was removed and the crude was dissolved in THF (110 ml). This solution was added to a solution of 4-amino benzoic acid (7.1 g, 51 mmol) and sodium carbonate (5.5 g, 51 mmol) in water (200 ml) and THF (100 ml). The mixture was stirred overnight at room temperature, then THF was removed under reduced pressure and the solution was treated with 1N HCl (102 ml, 102 mmol). The precipitate was filtered, dried under reduced pressure, tritured in diethyl ether and filtered yielding 13.2 g (yield 64%) of pure 4-[6-(diethylaminomethyl)naphth-2-ylmethyloxycarbamoyl]-benzoic acid; m.p.=201-205° C. (dec.)
1 H-NMR d 10.26 (s, 1H), 8.13 (s, 1H), 8.05-7.75 (m, 6H), 7.63 (m, 3H), 5.40 (s, 2H), 4.32 (s, 2H), 2.98 (q, 4H), 1.24 (t, 6H).
D. A solution of 4-[6-(diethylaminomethyl)naphth-2-ylmethyloxycarbamoyl]benzoic acid (13.1 g, 32 mmol) and thionyl chloride (7 ml, 96 mmol) in chloroform (300 ml) was refluxed for 4 hours, then the solvent and thionyl chloride were evaporated. Crude was dissolved in chloroform (100 ml) and evaporated to dryness three times. Crude was added as solid to a solution of hydroxylamine hydrochloride (2.7 g, 39 mmol) and sodium bicarbonate (5.4 g, 64 mmol) and 1N sodium hydroxide (39 ml, 39 mmol) in water (150 ml) and THF (50 ml). The mixture was stirred overnight at room temperature, then THF was removed under reduced pressure and the aqueous phase was extracted with ethyl acetate (3×100 ml). The combined organic phases were dried over anhydrous sodium sulphate and the solvent was removed under reduced pressure. Crude was dissolved in THF and treated with a 1.5 N etheric solution of HCl. The solid product was filtered and dried yielding 6 g (yield 41%) of pure 4-[6-(diethylaminomethyl)naphth-2-ylmethyloxycarbamoyl]benzohydroxamic acid hydrochloride as white solid; m.p.=162-165° C., (dec.)
1 H-NMR d 11.24 (s, 1H, exchange with D2 O), 10.88 (s, 1H, exchange with D2 O), 10.16 (s, 1H), 8.98 (bs, 1H, exchange with D2 O), 8.21 (s, 1H), 8.10-7.97 (m, 3H), 7.89 (d, 1H), 7.80-7.55 (m, 5H), 5.39 (s, 2H), 4.48 (d, 2H), 3.09 (m, 4H), 1.30 (t, 6H).

Some nmr predictions
CAS NO. 497833-27-9, [6-(diethylaminomethyl)naphthalen-2-yl]methyl N-[4-(hydroxycarbamoyl)phenyl]carbamate H-NMR spectral analysis
![[6-(diethylaminomethyl)naphthalen-2-yl]methyl N-[4-(hydroxycarbamoyl)phenyl]carbamate NMR spectra analysis, Chemical CAS NO. 497833-27-9 NMR spectral analysis, [6-(diethylaminomethyl)naphthalen-2-yl]methyl N-[4-(hydroxycarbamoyl)phenyl]carbamate H-NMR spectrum](http://pic11.molbase.net/nmr/nmr_image/2015-01-20/001/566/1566912_1h.png)

13 C NMR PREDICTIONS
![[6-(diethylaminomethyl)naphthalen-2-yl]methyl N-[4-(hydroxycarbamoyl)phenyl]carbamate NMR spectra analysis, Chemical CAS NO. 497833-27-9 NMR spectral analysis, [6-(diethylaminomethyl)naphthalen-2-yl]methyl N-[4-(hydroxycarbamoyl)phenyl]carbamate C-NMR spectrum](http://pic11.molbase.net/nmr/nmr_image/2015-01-20/001/566/1566912_13c.png)

COSY NMR…..http://www.nmrdb.org/

HMBC /HSQC

References![]()
- World Health Organization (2010). “International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended INN: List 63″. WHO Drug Information 24 (1): 58–9.
- 2
- National Cancer Institute (2010). “Gavinostat”. NCI Cancer Dictionary. U.S. National Institutes of Health. Retrieved 2010-09-15.
- 3
- “Search results for ITF2357″. ClinicalTrials.gov.
- 4
- Committee for Orphan Medicinal Products (23 February 2010). “Public summary of opinion on orphan designation: Givinostat for the treatment of systemic-onset juvenile idiopathic arthritis”. European Medicines Agency. Retrieved 2010-09-15.
- 5
- Committee for Orphan Medicinal Products (3 March 2010). “Public summary of opinion on orphan designation: Givinostat for the treatment of polycythaemia vera”. European Medicines Agency.
- 6
- WO patent application 1997/043251, “Compounds with anti-inflammatory and immunosuppressive activities”, published 1997-11-20, assigned to Italfarmaco S.p.A.
- 7
- Leoni F, Fossati G. (2005). “The histone deacetylase inhibitor ITF2357 reduces production of pro-inflammatory cytokines in vitro and systemic inflammation in vivo”. Molecular Medicine 11: 1. doi:10.2119/2006-00005.Dinarello. PMID 16557334.
- 8
- Tan J, Cang S, Ma Y, Petrillo RL, Liu D (2010). “Novel histone deacetylase inhibitors in clinical trials as anti-cancer agents”. Journal of Hematology & Oncology 3: 5. doi:10.1186/1756-8722-3-5. PMID 20132536. Review.
- 9
- Vannucchi AM, Guglielmelli P, Pieri L, Antonioli E, Bosi A (2009). “Treatment options for essential thrombocythemia and polycythemia vera.” Expert Review of Hematology 2 (1): 41–55. doi:10.1586/17474086.2.1.41. Review.
- Guerini V, Barbui V, Spinelli O, et al. (April 2008). “The histone deacetylase inhibitor ITF2357 selectively targets cells bearing mutated JAK2(V617F)”. Leukemia 22 (4): 740–7. doi:10.1038/sj.leu.2405049. PMID 18079739.
Further reading
- Job-Deslandre, C (January 2007). “Idiopathic juvenile-onset systemic arthritis”. Orphanet. Orphan number: ORPHA85414.
| US6034096 | 12 May 1997 | 7 Mar 2000 | Italfarmaco S.P.A. | Compounds with anti-inflammatory and immunosuppressive activities |
| WO1997043251A1 | May 12, 1997 | Nov 20, 1997 | Italfarmaco Spa | Compounds with anti-inflammatory and immunosuppressive activities |
| WO2004063146A1 | Jan 7, 2004 | Jul 29, 2004 | Italfarmaco Spa | Hydroxamic acid derivatives having anti-inflammatory action |
| WO2004065355A1 | Jan 8, 2004 | Aug 5, 2004 | Italfarmaco Spa | Monohydrate hydrochloride of the 4-hydroxycarbamoyl-phenyl)-carbamic acid (6-diethylaminomethyl-naphtalen-2-yl) ester |
| WO2006003068A2 | Jun 7, 2005 | Jan 12, 2006 | Italfarmaco Spa | Alpha-amino acid derivatives with antiinflammatory activity |
| WO2008097654A1 | Feb 8, 2008 | Aug 14, 2008 | Nancie M Archin | Methods of using saha for treating hiv infection |
| Citing Patent | Filing date | Publication date | Applicant | Title |
|---|---|---|---|---|
| US8518988 * | 3 Dec 2010 | 27 Aug 2013 | Chemi Spa | Polymorph of the hydrochloride of the (4-hydroxycarbamoyl-phenyl)-carbamic acid (6-dimethylamino methyl-2-naphthalenyl) ester |
| US20120302633 * | 3 Dec 2010 | 29 Nov 2012 | Chemi Spa | Novel polymorph of the hydrochloride of the (4-hydroxycarbamoyl-phenyl)-carbamic acid (6-dimethylamino methyl-2-naphthalenyl) ester |
| WO2011092556A1 | 3 Dec 2010 | 4 Aug 2011 | Chemi Spa | Novel polymorph of the hydrochloride of the (4-hydroxycarbamoyl-phenyl)-carbamic acid (6-dimethylamino methyl-2-naphtalenyl) ester |
 |
|
| Systematic (IUPAC) name | |
|---|---|
| {6-[(diethylamino)methyl]naphthalen-2-yl}methyl [4-(hydroxycarbamoyl)phenyl]carbamate | |
| Clinical data | |
|
|
| Legal status |
|
| Routes | Oral |
| Identifiers | |
| CAS number | 497833-27-9  |
| ATC code | None |
| PubChem | CID 9804992 |
| ChemSpider | 7980752  |
| UNII | 5P60F84FBH  |
| Chemical data | |
| Formula | C24H27N3O4 |
| Molecular mass | 421.489 g/mol |

| Italfarmaco S.p.A. | |
|---|---|
| Stato |  Italia Italia |
| Tipo | Società per azioni |
| Fondazione | 1938 a Milano |
| Fondata da | Gastone De Santis |
| Sede principale | Milano |
| Filiali |  Spagna – Spagna –  Portogallo Portogallo Grecia – Grecia –  Russia Russia Cile – Cile –  Brasile Brasile Turchia Turchia |
| Persone chiave | Francesco De Santis, [Presidente Holding] |
| Settore | sanità |
| Prodotti | Farmaci |
| Fatturato | >500 milioni di Euro (gruppo) (2011) |
| Dipendenti | >1900 (gruppo) (2011) |
| Sito web | www.italfarmaco.com |
MILAN ITALY


Filed under: 0rphan drug status, Phase2 drugs, Uncategorized Tagged: GIVINOSTAT, Italfarmaco, Italfarmaco S.p.A., juvenile idiopathic arthritis, juvenile rheumatoid arthritis, Orphan Drug, phase 2







