
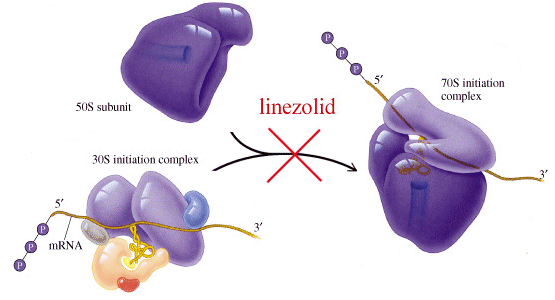
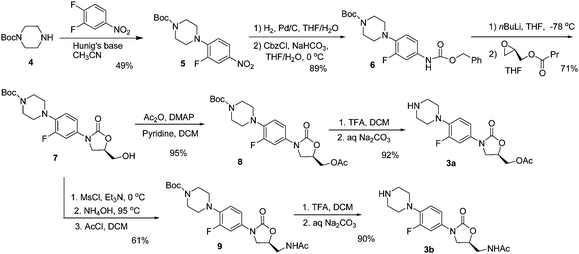
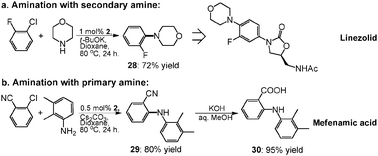
LINEZOLID
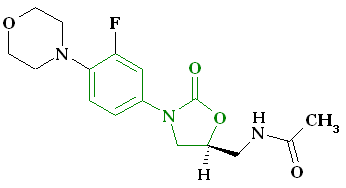
(S)-N-[[3-(3-fluoro-4-morpholinylphenyl)-2-oxo-5-oxazolidinyl]methyl] acetamide.
| N-[[(5s)-3-(3-fluoro-4-morpholin-4-ylphenyl)-2-oxo-1,3-oxazolidin-5-yl]methyl]acetamide PRODUCT PATENT US 5688792 (1997 to Pharmacia & Upjohn) |
|
| CAS No.: | 165800-03-3 |
|---|---|
| Synonyms: | |
| Formula: | C16H20FN3O4 |
| Exact Mass: | 337.14400 |
13C
1H NMR AND 13C PREDICT
1H NMR PREDICT
![N-[[(5S)-3-(3-fluoro-4-morpholin-4-ylphenyl)-2-oxo-1,3-oxazolidin-5-yl]methyl]acetamide NMR spectra analysis, Chemical CAS NO. 165800-03-3 NMR spectral analysis, N-[[(5S)-3-(3-fluoro-4-morpholin-4-ylphenyl)-2-oxo-1,3-oxazolidin-5-yl]methyl]acetamide H-NMR spectrum](http://pic11.molbase.net/nmr/nmr_image/2014-07-19/000/030/236/165800-03-3-1h.png)
13C NMR PREDICT
![N-[[(5S)-3-(3-fluoro-4-morpholin-4-ylphenyl)-2-oxo-1,3-oxazolidin-5-yl]methyl]acetamide NMR spectra analysis, Chemical CAS NO. 165800-03-3 NMR spectral analysis, N-[[(5S)-3-(3-fluoro-4-morpholin-4-ylphenyl)-2-oxo-1,3-oxazolidin-5-yl]methyl]acetamide C-NMR spectrum](http://pic11.molbase.net/nmr/nmr_image/2014-07-19/000/030/236/165800-03-3-13c.png)
COSY
PREDICT
HMBC
ORIGINAL 1H NMR…………...http://www.selleckchem.com/products/Linezolid(Zyvox).html
INTERMEDIATES USED
Arkivoc, , vol. 2012, # 6 p. 45 – 56
WO2011/137222 A1, ;

Union Quimico Farmaceutica, S.A. (UQUIFA) Patent: EP2163547 A1, 2010 ; Location in patent: Page/Page column 11 ;

THE REGENTS OF THE UNIVERSITY OF CALIFORNIA; GARG, Neil K.; RAMGREN, Stephen D.; SILBERSTEIN, Amanda L.; QUASDORF, Kyle W. Patent: WO2012/94622 A2, 2012 ; Location in patent: Page/Page column 31-32 ;

Lianhe Chemical Technology Co., Ltd. Patent: EP2388251 A1, 2011 ; Location in patent: Page/Page column 11 ;

Tammana, Rajesh; Vemula, Kiran Kumar; Guruvindapalli, Ramadasu; Yanamandr, Ramesh; Gutta, Madhusudhan Arkivoc, 2012 , vol. 2012, # 6 p. 45 – 56

Union Quimico Farmaceutica, S.A. (UQUIFA) Patent: EP2163547 A1, 2010 ; Location in patent: Page/Page column 10 ;

Song, Lirong; Chen, Xiaobei; Zhang, Shilei; Zhang, Haoyi; Li, Ping; Luo, Guangshun; Liu, Wenjing; Duan, Wenhu; Wang, Wei Organic Letters, 2008 , vol. 10, # 23 p. 5489 – 5492

Union Quimico Farmaceutica, S.A. (UQUIFA) Patent: EP2163547 A1, 2010 ; Location in patent: Page/Page column 10 ;

JUBILANT LIFE SCIENCES LIMITED; BISWAS, Sujay; PANDA, Atulya, Kumar; GUPTA, Ashish, Kumar; SINGH, Shishupal; TIWARI, Praveen; VIR, Dharam; THOMAS, Saji Patent: WO2013/111048 A1, 2013 ; Location in patent: Page/Page column 24; 25 ;

Perrault, William R.; Pearlman, Bruce A.; Godrej, Delara B.; Jeganathan, Azhwarsamy; Yamagata, Koji; Chen, Jiong J.; Lu, Cuong V.; Herrinton, Paul M.; Gadwood, Robert C.; Chan, Lai; Lyster, Mark A.; Maloney, Mark T.; Moeslein, Jeffery A.; Greene, Meredith L.; Barbachyn, Michael R. Organic Process Research and Development, 2003 , vol. 7, # 4 p. 533 – 546

US6362334 B1, ; Example 13 ;
NMR OF INTERMEDTIATES
-
[0003]The marketed pharmaceutical compositions are a sterile isotonic solution for an i.v. infusion, a tablet for oral administration and an aqueous suspension for oral administration. They are marketed, i.e., under brand name ZYVOX by Pfizer.
-
[0004]The molecule of linezolid has one asymmetric carbon in the molecule allowing for 2 enantiomers; the marketed compound is the (S)-enantiomer. In the above-marketed compositions, linezolid is present as a free base.
-
[0005]Hereinunder, the name linezolid will be used as the generic name for N-(3-(3-fluoro-4-(morpholin-4-yl)phenyl)-2-oxooxazolidin-5(S)-ylmethyl)acetamide, unless indicated to the contrary.
-
[0006]Linezolid was first disclosed in WO 95/07271 ( EP 0717738 , US 5,688,792 ) of the Upjohn Company.
-
[0007]Various processes for making linezolid are known in the art. In particular, the important ones are these, the final step of which comprises acetylation of an amine precursor of the formula (II) with an acetylhalide or acetic anhydride (see, e.g., WO 2005 099353 ),
-
[0008]This amine precursor (II) may be made from various starting materials, e.g.:
- a) By a reduction of an azide compound of formula (III) by a suitable reductant ( WO2006/091731 , WO 95/07271 , US 5837870 , WO2009/063505 , US 7291614 ),
The starting compound (III) may be made from the corresponding tosylate or chloride of general formula (VII) below ( WO 2005/099353 ).
- b) By a decomposition of a phthalimide compound of formula (IV), e.g. by methylamine ( WO95/07271 ) or by hydrazine ( US 5837870 ),
The starting compound (IV) may be made from the same tosylate or chloride as sub a) ( WO2005/099353 ) or by a cyclization of the oxazolidine ring ( WO 99/24393 , WO2006/008754 ).
- c) From a sulfonate compound of formula (V),
by treatment with ammonium hydroxide in isopropanol or THF ( WO 95/07271 ) or by treatment with ammonia under enhanced pressure ( WO 97/37980 ).
- d) By a reduction of an imine (VI),
wherein R2 is a chlorophenyl, bromophenyl or 2,4,-dichlorophenyl moiety ( WO 2007/116284 ).
- a) By a reduction of an azide compound of formula (III) by a suitable reductant ( WO2006/091731 , WO 95/07271 , US 5837870 , WO2009/063505 , US 7291614 ),
-
[0009]Except of the imine (VI), each of the preceded synthetic approaches is based on a step of converting a starting material of the general formula (VII),
wherein L is a suitable leaving group, for instance a halogen or an alkyl-or aryl sulfonyloxy group,
by a reaction with a nitrogen nucleophile (an azide salt, phthalimide salt, ammonia or ammonium hydroxide), followed, if necessary, by a next step of conversion of the formed reaction intermediate (e.g., compound (III) or compound (IV)) into the amino/compound (II). Apparently, making the starting amine-compound (II) in a good yield and purity is the key aspect of commercial success of any of the above synthetic routes yielding linezolid. However, the known approaches have various drawbacks, for instance serious toxicity and explosion hazard of the azide salts, long reaction times and hazardous agents (hydrazine, methyl amine) in using the phthalimide intermediate, low yields and many side products at the ammonium hydroxide approach, or harsh reaction conditions in reaction with ammonia.
is reacted without isolation with acetic anhydride as an oily product, or in solution, to produce the acetamide, linezolid (1). This is followed by procedures for isolating the linezolid (1) such as those described in U.S. Pat. No. 5,688,792, at col. 15, 11. 22-28 (chromatography and separation of the desired fraction, followed by evaporation and trituration of the product to obtain pure linezolid (1)).
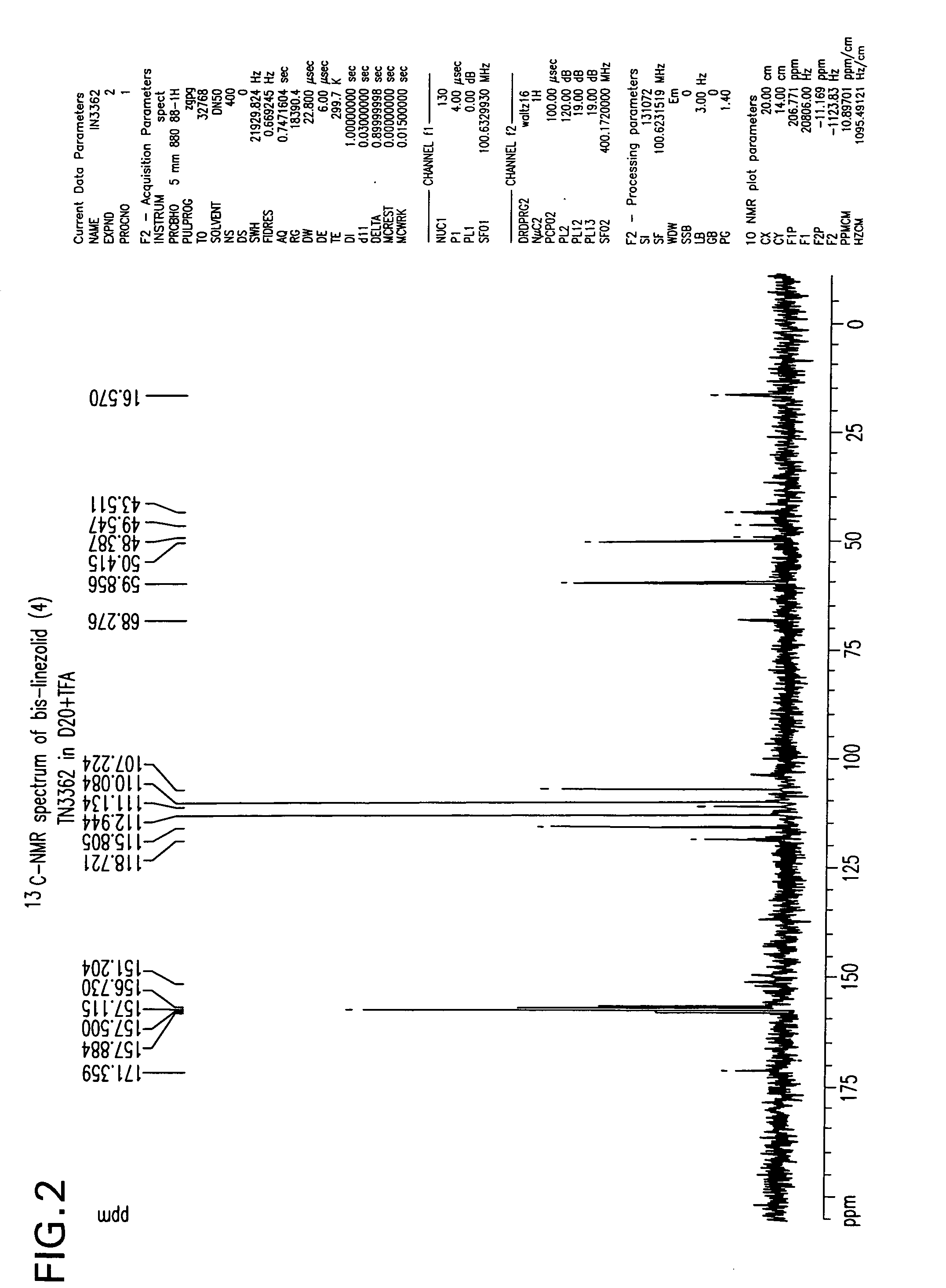
is reduced to its corresponding amine, S-N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (2) in the solvent ethyl acetate by hydrogenation using hydrogen gas and a palladium/carbon catalyst. These reaction conditions lead to the production of an undesirable level of reaction by-products, and, following the acetylation of the intermediate amine (2) to linezolid (1), to undesirably high levels of bis-linezolid (4)
http://www.google.com/patents/US20060252932


A Novel Synthesis of Oxazolidinone Derivatives (A Key Intermediate of Linezolid)
2Center for Pharmaceutical sciences, Jawaharlal Nehru Technological University, Kukatpally, Hyderabad, India
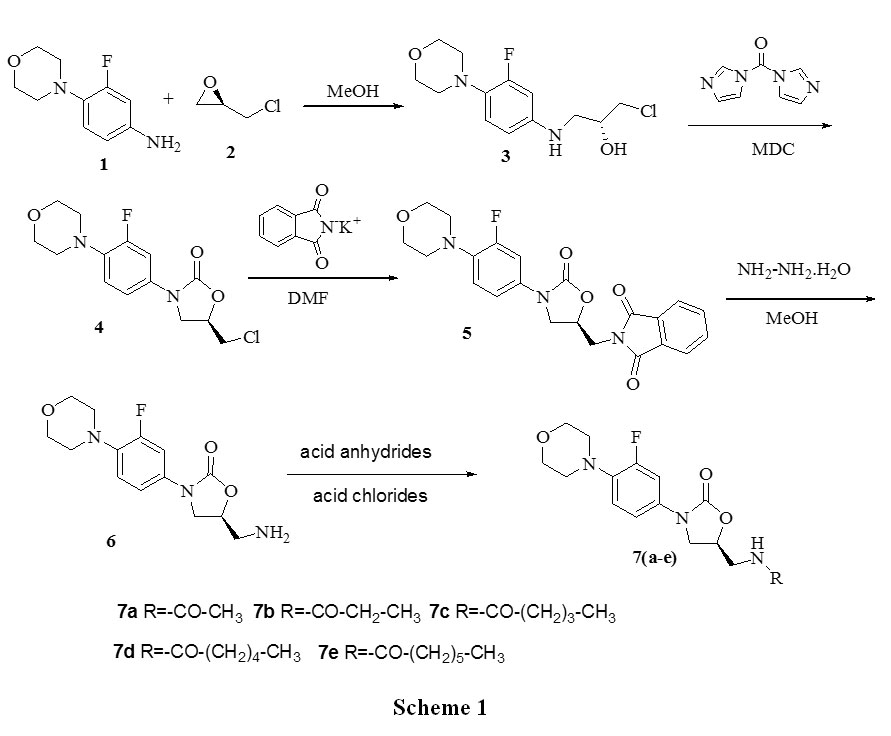
N-[[(5S)-3-[3-fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]acetamide (7a):
IR (KBr, cm-1): 3338 (N-H stretching), 3117, 3066 (aromatic C-H stretching), 2971, 2863, 2818 (aliphatic C-H stretching), 1738, 1662 (C=O stretching), 1545, 1516,1453 (aromatic C=C stretching), 1425 (C-N stretching), 1381 (aliphatic C-H bending), 1334 (C-F stretching), 1274 (C-O stretching), 1198, 1177 (C-N bending), 1117, 1081 (aromatic C-H bending).
1H NMR (CDCl3) δ ppm: 7.44 (m, 1H), 7.26 (m, 1H), 6.99 (m, 1H), 6.01 (t,1H), 4.76 (m, 1H), 4.02 (m, 2H), 3.80 (m, 4H), 3.61(m, 2H), 3.05 (m, 4H), 2.02 (t, 3H):
C13NMR(CDCl3) δppm: 171.33, 156.87, 154.44, 136.40, 132.84, 118.67, 113.81, 107.52, 71.96, 66.76, 50.79, 47.46, 41.68, 22.81. MS: 338 (M++H);
……………………………………………………………………….
ARKIVOC 2012 (vi) 45-56 Page 45 ©ARKAT-USA, Inc.
An expeditious construction of 3-aryl-5-(substituted methyl)-2- oxazolidinones: a short and efficient synthesis of Linezolid
Rajesh Tammana,a,b Kiran Kumar Vemula,a Ramadasu Guruvindapalli,a Ramesh Yanamandra,c and Madhusudhan Gutta* a
aDepartment of Research & Development, Inogent Laboratories Pvt. Ltd.,
A GVK BIO Company, 28A, IDA, Nacharam, Hyderabad 500 076, Andhra Pradesh, India
bCentre for Pharmaceutical Sciences, Institute of Science and Technology, Jawaharlal Nehru Technological University, Hyderabad 500 072, Andhra Pradesh, India
cDepartment of Analytical Research & Development, GVK Biosciences Pvt. Ltd., 28A, IDA, Nacharam, Hyderabad 500 076, Andhra Pradesh, India
E-mail: madhusudhan.gutta@inogent.com
http://www.arkat-usa.org/get-file/42622/
N-(((S)-3-(3-fluoro-4-morpholinophenyl)-2-oxooxazolidin-5-yl)methyl)acetamide 1 (Linezolid) 1 was prepared according to the method described in literature.12,15
Mp 182-183 °C, (lit.12a 181.5- 182.5 °C); enantiomeric purity 99.9% (by chiral HPLC);
IR (KBr): ν 3343 (NH), 3075 (Ar-H), 2967 (CH), 1741 (C═O), 1660 (C═O) cm-1 ;
1H NMR (CDCl3): δ 2.03 (s, 3H), 3.04-3.07 (t, 4H), 3.56-3.77 (m, 3H), 3.86-3.89 (t, 4H), 4.00-4.06 (t, 1H), 4.74-4.79 (m, 1H), 5.96 (s, 1H), 6.90- 6.96 (t, 1H), 7.06-7.10 (d, 1H), 7.43-7.48 (d, 1H).
13C NMR (DMSO-d6): δ 22.4, 41.4, 47.3, 50.6, 66.1, 71.5, 106.4, 114.0, 119.1, 133.3, 135.5, 154.0, 156.2, 170.0;
ESI-MS (C16H20FN3O4): m/z (%) 338.18 (100, M+ +1).
12. (a) Brickner, S. J.; Hutchinson, D. K.; Barbachyn, M. R.; Manninen, P. R.; Ulanowicz, D. A.;
Garmon, S. A.; Grega, K. C.; Hendges, S. K.; Toops, D. S.; Ford, C. W.; Zurenko, G. E. J.
Med. Chem. 1996, 39, 673. (b) Barbachyn, M. R.; Brickner, S. J.; Hutchinson, D. K. U.S.
patent 5688792; 1997; Chem. Abstr. 1995, 123, 256742. (c) Dhananjay, G. S.; Nandu, B. B.;
Avinash, V. N.; Kamlesh, D. S.; Anindya, S. B.; Tushar, A. N. PCT Int. Appl. 063505, 2009;
Chem. Abstr. 2009, 150, 515152.
13. (a) Imbordino, R. J.; Perrault, W. R.; Reeder, M. R. PCT Int. Appl. 116284, 2007; Chem.
Abstr. 2007, 147, 469356. (b) Pearlman, B. A.; Perrault, W. R.; Barbachyn, M. R.;
Manninen, P. R.; Toops, D. S.; Houser, D. J.; Fleck, T. J. U.S. Patent 5837870, 1998; Chem.
Abstr. 1998, 130, 25061. (c) Perrault, W. R.; Pearlman, B. A.; Godrej, D. B.; Jeganathan, A.;
Yamagata, K.; Chen, J. J.; Lu, C. V.; Herrinton, P. M.; Gadwood, R. C.; Chan, L.; Lyster, M.
A.; Maloney, M. T.; Moeslein, J. A.; Greene, M. L.; Barbachyn, M. R. Org. Proc. Res. Dev.
2003, 7, 533.
14. (a) Yu, D. S.; Huang, L.; Liang, H.; Gong, P. Chin. Chem. Lett. 2005, 16, 875. (b) Pearlman,
B. A. PCT Int. Appl. 9924393, 1999; Chem. Abstr. 1999, 130, 338099. (c) Weigert, F. J. J.
Org. Chem. 1973, 38, 1316.
15. (a) Wang, M.; Tong, H. CN patent 101220001, 2008. (b) Mohan Rao, D.; Krishna Reddy, P.
PCT Int. Appl. 099353, 2005; Chem. Abstr. 2005, 143, 440395. (c) Mohan Rao, D.; Krishna
Reddy, P. PCT Int. Appl. 008754, 2006; Chem. Abstr. 2006, 144, 170978.
……………………………………
Organic Process Research and Development, 2003 , vol. 7, # 4 p. 533 – 546
http://pubs.acs.org/doi/abs/10.1021/op034028h

(S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo- 5-oxazolidinyl]methyl]acetamide: Linezolid: Zyvox
HPLC analyses showed the first and second crops to be 98.9 and 94.6 wt % linezolid, respectively, with <0.2% enantiomer in each; also, an additional 9.7% yield of linezolid was detected in the filtrate by external standard HPLC (total ) 80.6%). Analysis data for 1st crop material: mp ) 73-76 °C;
1 H NMR (CDCl3, 400 MHz)
δ 7.43 (dd, J ) 14.4, 2.4 Hz, 1H), 7.07 (dd, J ) 8.8, 2.0 Hz, 1H), 6.91 (t, J ) 8.8 Hz, 1H), 6.43 (br t, 1H), 4.77 (m, 1H), 4.02 (t, J ) 9.2 Hz, 1H), 3.86 (t, J ) 4.4 Hz, 4H), 3.76 (dd, J ) 8.8, 6.8 Hz, 1H), 3.66 (m, 2H), 3.05 (t, J ) 4.8 Hz, 4H), 2.02 (s, 3H);
13C NMR (CDCl3, 100 MHz)
δ 23.07 (q), 41.93 (t), 47.66 (t), 51.00 (t), 66.95 (t), 71.99 (d), 107.56 (dd, JC-F ) 26.16 Hz), 113.97 (dd, JC-F ) 3.02 Hz), 118.85 (dd, JC-F ) 4.03 Hz), 132.90 (sd, JC-F ) 4.03 Hz), 136.58 (sd, JC-F ) 9.06 Hz), 154.42 (s), 155.50(sd, JC-F ) 246.53 Hz), 171.19 (s)
MS (EI) m/z (relative intensity) 337 (90), 293 (81), 209 (100);
[R]25D ) -16 (c ) 1.05, ethanol).
Anal. Calcd for C16H20FN3O4: C, 56.97; H, 5.97; N, 12.46; found: C, 56.86; H, 6.05; N, 12.44
HPLC (99.0 wt %, 98.9 area % linezolid, tR 1.60 min) conditions: InertsilODS-2 5.0 µm 150 mm × 4.6 mm, flow rate ) 2.0 mL/ min, gradient elution from 40:60 A:B to 80:20 A:B over 10 min; A ) acetonitrile; B ) water. External standard HPLC analysis of the filtrate showed
d 12.9% and 7.6% yield of linezolid and 8, respectively.
SEE HPLC AT http://file.selleckchem.com/downloads/hplc/S140801-Linezolid-Zyvox-HPLC-Selleck.pdf
………………………….
http://www.google.com/patents/WO2007064818A1?cl=en
Linezolid
Desfluoro Linezolid
HTTP://WWW.GOOGLE.COM/PATENTS/US6559305
HTTP://WWW.GOOGLE.COM/PATENTS/US7989618
……………………………………….
http://www.google.com/patents/EP2690100A1?cl=en
Example 3
-
[0034]To a 25 ml, round-bottomed flask equipped with a magnetic stirring bar was charged “amine” (0.49 g) followed by water (8.30 ml). A heterogeneous mixture was stirred and hydrochloric acid (0.12 mL, 35 %) was added. A homogenous solution was obtained. The solution was cooled down in an ice-water bath to 0°C. Acetic anhydride (0.31 mL) was added followed by sodium bicarbonate (0.45 g). Carbon dioxide was immediately released and a formation of white precipitate was observed. The precipitate was filtered off and the filter cake was washed with water (10 ml). The filter cake was collected and dried (100 mbar) at 70°C overnight. An off-white solid linezolid (0.26 g) was isolated.
…………………………..
PATENT
http://www.google.com/patents/WO2007116284A1?cl=en
Example 4 Trituration (convert linezolid crystalline Form I to linezolid crystalline Form E) The product from Example (89.18 g) is transferred to a 3L round bottom flask equipped with a mechanical stirrer, thermocouple and heating mantel. Ethyl acetate (2.23 L, 15 mL/g) is added and seeded with Linezolid form II crystals and the slurry is heated to ca. 500C. A slight exotherm of 30C is observed. After 30 minutes of heating the form change is observable as the solid is changing to long needles. Stirring is continued for 2 hours at 500C, at which time the contents are cooled to ambient temperature and stirred for an additional 30 minutes. The contents are then cooled to 30C for 1.5 hours, filtered and washed with cold ethyl acetate (300 mL total). The resultant solids are dried under vacuum at 50°C for 18 hours to give Linezolid (78.12 g) Form II by XRD, 99.8 wt%, 99.9% ee. HPLC conditions: YMC 5μ ODS-AM 150 nm X 4.6 nm column, etuting with CH3CN /water + 0.1% TFA from 20% CH3CN to 80% CH3CN in 8 min at 0.5 mL/min, detecting at 254nm. TR (Linezolid) = 4.4 min; HPLC conditions: Chiralcel OJ-H 250 nm X 4.6 nm column, eluting with 90% CO2/ 10%MeOH at 3.0 mL/min, detecting at 255 nm. TR [title compound] = 3.6 min; TR (enantiomer of title compound) = 4.1 rain
……………………………………..
http://www.google.com/patents/EP2516408A1?cl=en
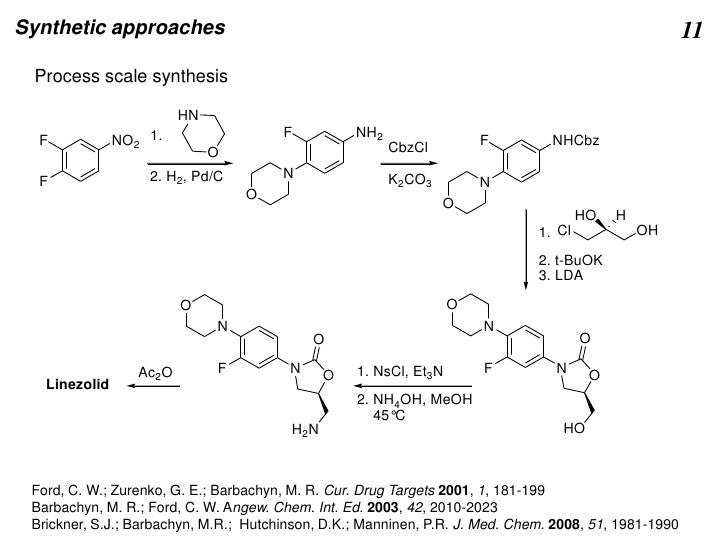
The polymorphic form obtained by following process disclosed in U.S. Pat. No. 5,688,792 is designated as Form I. Figure- 1 depicts the PXRD graph of Form I obtained by following prior art process. [15] Disadvantage of the process disclosed in U.S. Pat. No. 5,688,792 is that it involves use of n-butyl lithium. Due to its explosive nature it is difficult to handle at plant scale. Also, the said reaction is carried out at temperature of -78°C, which is difficult to attain during commercial production. Further the intermediate obtained requires purification by column chromatography. Column chromatography is a cumbersome technique and difficult to practice during commercial scale production.
The process for the preparation of Linezolid is also disclosed in Journal of Medicinal Chemistry (1996), 39(3), 673-9, U.S. Pat. Nos. 6,492,555, 5,837,870, 6,887,995, 7,307,163, 7,429,661, etc.
Linezolid was first disclosed in U.S. Pat. No. 5,688,792. The process for synthesis is as disclosed in Scheme-I


………………………………………..
https://acs.confex.com/acs/green08/techprogram/P52019.HTM
Wednesday, June 25, 2008 – 2:00 PM
New York (Capital Hilton)
128
Convergent Green Synthesis of Linezolid (Zyvox)
………………………………….

……………………………………..

……………………………………….
http://www.google.com/patents/EP2072505A2?cl=en
-
[0003]Other synthetic routes for the preparation of linezolid are reported for example in US 6107519 and in Tetrahedron Letters, Vol 37, N° 44, pages 7937-7940, wherein the chiral compound shown below is used instead of glycidyl butyrate as a synthon containing the molecule stereocenter.
-
[0004]It should be appreciated that all of the known approaches to the preparation of linezolid make use of chiral synthons for the construction of the stereocenter. These are small molecules characterized by a high cost, therefore they are not suitable for the production of the compound on an industrial scale.
-
[0005]There is therefore the need for an alternative synthesis which provides oxazolidinone derivatives, linezolid included, from inexpensive starting materials, and which does not require a chiral synthon for the construction of the molecule, so that it can be used for the industrial preparation of such derivatives.
………………………………….
http://pubs.rsc.org/en/content/articlelanding/2010/md/c0md00015a/unauth
………………………………….

…………………………………………
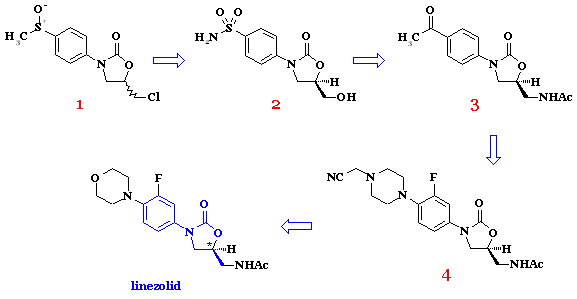
…………………………………
DOI: 10.1039/C3RA45186K
http://pubs.rsc.org/en/content/articlelanding/2013/ra/c3ra45186k#!divAbstract

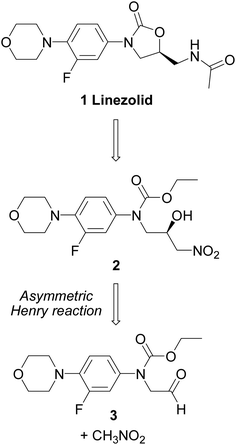
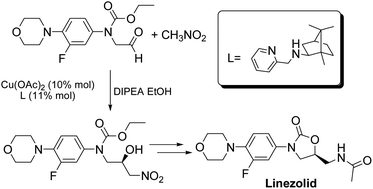
A new asymmetric synthesis of the antibiotic Linezolid was performed through a copper-catalyzed Henry reaction as the key step. The use of camphor-derived aminopyridine ligands helped to improve the yields of the chiral precursor and to obtain Linezolid in good overall yield and enantiomeric excess.
Linezolid 1. Mp: 181–182 C [lit. 181.5–182.5 C];
1 H-NMR (300 MHz; CDCl3) d 2.02 (s, 3H), 3.06 (t, J ¼ 4.7 Hz, 4H), 3.61– 3.78 (m, 3H), 3.87 (t, J ¼ 4.7 Hz, 4H), 4.03 (t, J ¼ 9.0 Hz, 1H), 4.72–4.82 (m, 1H), 6.17 (bt, 1H, exch. with D2O), 6.93 (t, J ¼ 9.0 Hz, 1H), 7.08 (dd, J1 ¼ 9.0 Hz, J2 ¼ 2.5 Hz, 1H), 7.44 (dd, J1 ¼ 14.4 Hz, J2 ¼ 2.5 Hz, 1H); ee ¼ 71%;
HPLC (Daicel CHIRALPAK-IA, hexane/i-PrOH ¼ 70 : 30, ow rate 0.8 mL min 1 , l ¼ 254 nm); tR (major) ¼ 14.1 min; tR (minor) ¼ 16.4 min. A true sample of (S)-Linezolid (ee > 98%) under the same HPLC conditions gave a tR ¼ 14.1 min.
………………………………..
http://www.slideshare.net/vishwajeeta/introduction-new-ppt
………………………………
http://www.slideshare.net/pushechnikov/linezolid-case-study
…………………………………
http://pubs.rsc.org/en/content/articlelanding/2011/cc/c1cc15503b#!divAbstract
…………………………………
http://www.mdpi.com/1424-8247/3/7/1988/htm
………………………………

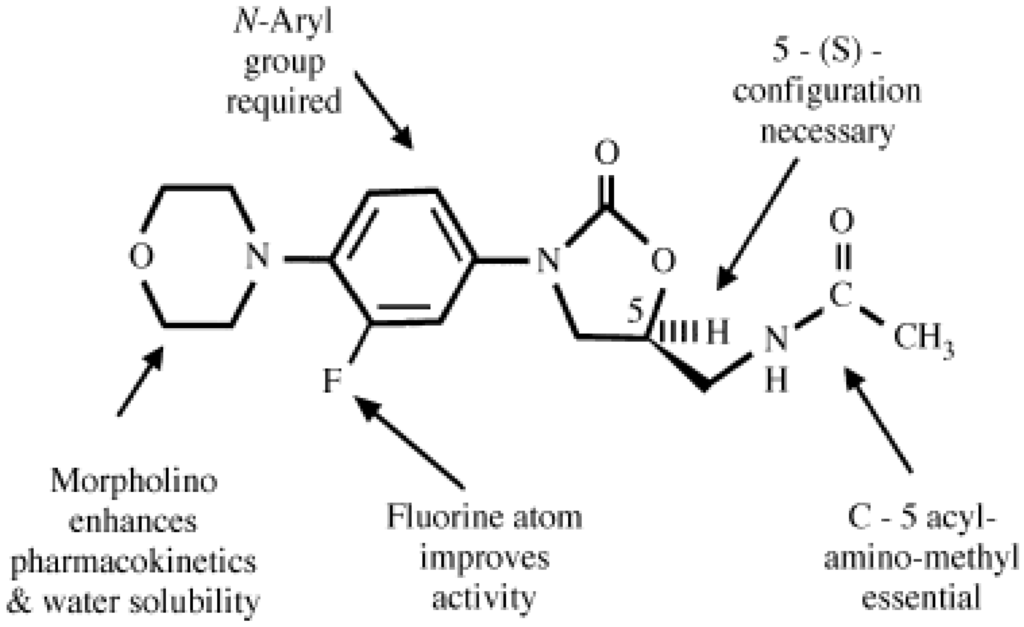
Numbered structure of linezolid, showing the pharmacophore required for good activity (in blue) and desirable structural features (in orange).
 |
|
 |
|
| Systematic (IUPAC) name | |
|---|---|
| (S)-N-({3-[3-fluoro-4-(morpholin-4-yl)phenyl]-2-oxo-1,3-oxazolidin-5-yl}methyl)acetamide | |
| Clinical data | |
| Trade names | Zyvox, Zyvoxam, Zyvoxid |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a602004 |
| Licence data | US FDA:link |
|
|
|
|
| Intravenous infusion, oral | |
| Pharmacokinetic data | |
| Bioavailability | ~100% (oral) |
| Protein binding | Low (31%) |
| Metabolism | Hepatic (50–70%, CYPnot involved) |
| Half-life | 4.2–5.4 hours (shorter in children) |
| Excretion | Nonrenal, renal, and fecal |
| Identifiers | |
165800-03-3  |
|
| J01XX08 | |
| PubChem | CID 441401 |
| DrugBank | DB00601  |
| ChemSpider | 390139  |
| UNII | ISQ9I6J12J  |
| KEGG | D00947  |
| ChEMBL | CHEMBL126  |
| NIAID ChemDB | 070944 |
| Chemical data | |
| Formula | C16H20FN3O4 |
| 337.346 g/mol | |
| Cited Patent | Filing date | Publication date | Applicant | Title |
|---|---|---|---|---|
| WO1995007271A1 * | Aug 16, 1994 | Mar 16, 1995 | Michael R Barbachyn | Substituted oxazine and thiazine oxazolidinone antimicrobials |
| AU2001100437A4 * | Title not available | |||
| EP0963980A2 * | Mar 10, 1999 | Dec 15, 1999 | The Wellcome Foundation Limited | 1,2,4-Triazine derivative, its preparation and its use as reference marker for testing purity and stability of “lamotrigine” |
| Reference | ||
|---|---|---|
| 1 | * | [Online] August 2002 (2002-08), XP002388488 Retrieved from the Internet: URL:www.emea.eu.int/pdfs/human/ich/273799e n.pdf> [retrieved on 2006-07-03] |
| 2 | * | [Online] June 1995 (1995-06), XP002388489 Retrieved from the Internet: URL:www.emea.eu.int/pdfs/human/ich/38195en .pdf> [retrieved on 2006-07-03] |
| 3 | * | DATABASE CA [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; LIU, JUN ET AL: “Preparation of oxazolidone derivatives as antibacterial agents” XP002429969 retrieved from STN Database accession no. 2003:576097 -& CN 1 355 165 A (INSTITUTE OF MEDICAL AND BIOLOGICAL TECHNOLOGY, CHINESE ACADEMY OF MED) 26 June 2002 (2002-06-26) |
| 4 | * | GLEAVE D M ET AL: “Synthesis and antibacterial activity of [6,5,5] and [6,6,5] tricyclic fused oxazolidinones” BIOORGANIC & MEDICINAL CHEMISTRY LETTERS, OXFORD, GB, vol. 8, no. 10, 19 May 1998 (1998-05-19), pages 1231-1236, XP004137053 ISSN: 0960-894X |
| 5 | * | REDDY K V S R K ET AL: “Isolation and characterization of process related impurities in linezolid” JOURNAL OF PHARMACEUTICAL AN BIOMEDICAL ANALYSIS, vol. 30, no. 3, 15 October 2003 (2003-10-15), pages 635-642, XP002388486 |
| WO2001057035A1 * | Jan 29, 2001 | Aug 9, 2001 | Upjohn Co | Linezolid-crystal form ii |
| WO2002032857A1 * | Oct 17, 2001 | Apr 25, 2002 | Robert C Gadwood | Methods of producing oxazolidinone compounds |
| WO2002085849A2 * | Apr 15, 2002 | Oct 31, 2002 | Delara B Godrej | Process to prepare oxazolidinones |
| WO2005099353A2 * | Apr 19, 2004 | Oct 27, 2005 | Reddy Pingili Krishna | A novel process for the preparation of linezolid and related compounds |
| WO2006008754A1 | Jul 20, 2004 | Jan 26, 2006 | Reddy Pingili Krishna | Novel intermediates for linezolid and related compounds |
| WO2006031179A1 * | Sep 12, 2005 | Mar 23, 2006 | Astrazeneca Ab | Process for preparation of phtalimide |
| WO2007116284A1 * | Mar 26, 2007 | Oct 18, 2007 | Pfizer Prod Inc | Process for preparing linezolid |
| WO2010081404A1 | Jan 8, 2010 | Jul 22, 2010 | Lianhe Chemical Technology Co., Ltd. | Method for preparing linezolid and intermediates thereof |
| WO2012019632A1 | Aug 11, 2010 | Feb 16, 2012 | Synthon B.V. | Process for making linezolid |
| WO2012019862A1 | Jul 14, 2011 | Feb 16, 2012 | Synthon B.V. | Process for making linezolid |
| WO2012114354A1 | Feb 21, 2012 | Aug 30, 2012 | Lee Pharma Limited | Anhydrous linezolid crystalline form-ii |
| WO2013072923A1 | Sep 18, 2012 | May 23, 2013 | Cadila Healthcare Limited | Process for the preparation of crystalline linezolid |
| WO2013111048A1 | Jan 22, 2013 | Aug 1, 2013 | Jubilant Life Sciences Limited | Improved process for the preparation of stable crystalline form-i of linezolid, substantially free of residual solvent |
| WO2014071990A1 | Nov 9, 2012 | May 15, 2014 | Synthon Bv | Process for making linezolid |
| EP1403267A1 * | Sep 25, 2003 | Mar 31, 2004 | Daiso Co., Ltd. | Process for preparing glycidylphthalimide |
| EP1564215A1 * | Sep 25, 2003 | Aug 17, 2005 | Daiso Co., Ltd. | Process for preparing glycidylphthalimide |
| EP2100884A1 | Oct 16, 2003 | Sep 16, 2009 | Symed Labs Limited | Crystalline form of linezolid |
| EP2690100A1 | Jul 14, 2011 | Jan 29, 2014 | Synhton B.V. | Process for making linezolid |
| US6444813 | Jan 29, 2001 | Sep 3, 2002 | Pharmacia & Upjohn Company | Mixing linezolid of an >98% enantomeric purity in a solvent at >80 degrees; separating a crystal (ii) of >99% purity; analysis by the powder x-ray diffraction spectrum/infrared spectrum as a mineral oil mull; bactericides; stability |
| US6514529 | Mar 15, 2001 | Feb 4, 2003 | Pharmacia & Upjohn Company | A compressed tablet of antibacterial oxazolidinone selected from the group consisting of linezolid, eperezolid and (S)-N-((3-(3-fluoro-4-(tetrahydro-2H-thiopyran-4-yl)phenyl-2-o xo-5-oxazolidinylmethyl)acetamide S,S-dioxide |
| US6544991 | Jun 21, 2001 | Apr 8, 2003 | Pharmacia & Upjohn Company | Compositions and methods for treating bacterial infections |
| US6559305 | May 23, 2002 | May 6, 2003 | Pharmacia & Upjohn Company | Linezolid—crystal form II |
| US6617339 | Jun 3, 1999 | Sep 9, 2003 | Syngenta Limited | Oxazolidinone derivatives, process for their preparation and pharmaceutical compositions containing them |
| US6796975 | Mar 15, 2001 | Sep 28, 2004 | Pharmacia & Upjohn Company | Container for linezolid intravenous solution |
| US6833453 | Oct 17, 2001 | Dec 21, 2004 | Pharmacia & Upjohn Company | As an example, manufacturing a 5-(tert-butylcarbamoyl)-amino-methyl-oxazolidinone by condensing a carbamate with a tert-butylcarbamoyl protected derivative of glycidylamine or a 3-amino-1-halopropanol |
| US6875875 | Sep 25, 2003 | Apr 5, 2005 | Daiso Co., Ltd. | Process for preparing glycidylphthalimide |
| US6887995 | Apr 15, 2002 | May 3, 2005 | Pharmacia & Upjohn Company | Reacting N-aryl-O-alkylcarbamate with an amide derivative in the presence of a lithium cation, a base, and a nucleophile |
| US6989381 | Aug 20, 2001 | Jan 24, 2006 | Pharmacia Corporation | Containing s cyclodextrin compound in a concentration sufficient to maintain the drug in solution at such a drug concentration. |
| US7087784 | Mar 25, 2004 | Aug 8, 2006 | Pharmacia & Upjohn | Process to prepare oxazolidinones |
| US7128928 | Feb 20, 2003 | Oct 31, 2006 | Pharmacia Corporation | Ophthalmic formulation with novel gum composition |
| US7135576 | Jan 7, 2005 | Nov 14, 2006 | Daiso Co., Ltd. | Process for preparing glycidylphthalimide |
| US7307163 | Apr 19, 2004 | Dec 11, 2007 | Symed Labs Limited | Process for the preparation of linezolid and related compounds |
| US7351824 | Oct 8, 2007 | Apr 1, 2008 | Symed Labs Limited | Intermediates for oxazolidinone antibacterials; N-[3-Chloro-2-(R)-hydroxypropyl]-3-fluoro-4-morpholinyl aniline |
| US7429661 | Jul 20, 2004 | Sep 30, 2008 | Symed Labs Limited | Intermediates for linezolid and related compounds |
| US7524954 | Oct 8, 2007 | Apr 28, 2009 | Symed Labs Limited | Reacting 3-fluoro-4-morpholinyl aniline derivative with epichlorohydrin; converting chloromethyl oxazolidinone to aminomethyl oxazolidinone; carbonylation ; reacting with potassium phthalimide, hydrazine hydrate, and acetic anhydride; cyclization, carbamylation |
| US7714128 | Oct 16, 2003 | May 11, 2010 | Symed Labs Limited | crystalline linezolid form III (N-[[(5S)-3-[3-fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]acetamide) an antibacterial agent; thermal stability |
| US7718799 | Sep 26, 2007 | May 18, 2010 | Symed Labs Limited | Crystalline form of linezolid |
| US7718800 | Sep 26, 2007 | May 18, 2010 | Symed Labs Limited | Prepared by mixing linezolid with solvent or mixture of solvents, cooling contents to below 15 degrees C., optionally seeding contents with linezolid form III, stirring, and collecting linezolid form III crystals by filtration or centrifugation; antibacterial agent; thermally stable |
| US7732597 | Sep 26, 2007 | Jun 8, 2010 | Symed Labs Limited | Prepared by acetylating (S)-N-[[3-[3-fluoro-4-[4-morpholinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]amine in a solvent, optionally in presence of an organic base to form linezolid, seeding reaction mixture, and isolating linezolid form III; antibacterial agent; thermally stable |
| US7741480 | Oct 8, 2007 | Jun 22, 2010 | Symed Labs Limited | Process for the preparation of linezolid and related compounds |
| US8658789 | Jan 8, 2010 | Feb 25, 2014 | Lianhe Chemical Technology Co., Ltd. | Method for preparing linezolid and intermediates thereof |
| US5837870 | Mar 28, 1997 | Nov 17, 1998 | Pharmacia & Upjohn Company | Process to prepare oxazolidinones |
| US6107519 | Oct 13, 1998 | Aug 22, 2000 | Pharmacia & Upjohn Company | Amido-substituted secondary alcohol intermediates and preparation thereof |
| US6444813 | Jan 29, 2001 | Sep 3, 2002 | Pharmacia & Upjohn Company | Mixing linezolid of an >98% enantomeric purity in a solvent at >80 degrees; separating a crystal (ii) of >99% purity; analysis by the powder x-ray diffraction spectrum/infrared spectrum as a mineral oil mull; bactericides; stability |
| US6492555 | Jan 15, 2002 | Dec 10, 2002 | Pharmacia & Upjohn Company | Reaction of a carbamate with either a (s)-secondary alcohol or (s)-epoxide or (s)-ester; bactericides |
| US6559305 | May 23, 2002 | May 6, 2003 | Pharmacia & Upjohn Company | Linezolid—crystal form II |
| US6716980 | Jun 27, 2003 | Apr 6, 2004 | Pharmacia & Upjohn Company | Cyclization and acylation of carbamate |
| US6740754 | Apr 24, 2003 | May 25, 2004 | Pharmacia & Upjohn Company | Process to produce oxazolidinones |
| US6833453 | Oct 17, 2001 | Dec 21, 2004 | Pharmacia & Upjohn Company | As an example, manufacturing a 5-(tert-butylcarbamoyl)-amino-methyl-oxazolidinone by condensing a carbamate with a tert-butylcarbamoyl protected derivative of glycidylamine or a 3-amino-1-halopropanol |
| US6887995 | Apr 15, 2002 | May 3, 2005 | Pharmacia & Upjohn Company | Reacting N-aryl-O-alkylcarbamate with an amide derivative in the presence of a lithium cation, a base, and a nucleophile |
| US7649096 * | Jul 17, 2006 | Jan 19, 2010 | Glenmark Pharmaceuticals Limited | crystallization of linezolid antibacterial agent in solvent and antisolvent |
| US20060111350 | Jun 29, 2005 | May 25, 2006 | Judith Aronhime | Solid forms of linezolid and processes for preparation thereof |
| US20060142283 | Jun 29, 2005 | Jun 29, 2006 | Judith Aronhime | Crystalline form IV of linezolid |
| US20090156806 | Dec 11, 2008 | Jun 18, 2009 | Dipharma Francis S.R.I. | Process for the Preparation of Oxazolidinone Derivatives |
| WO1995007271A1 | Aug 16, 1994 | Mar 16, 1995 | Michael R Barbachyn | Substituted oxazine and thiazine oxazolidinone antimicrobials |
| WO2005035530A1 | Oct 16, 2003 | Apr 21, 2005 | Reddy Pingili Krishna | A novel crystalline form of linezolid |
| WO2007026369A1 | Aug 29, 2005 | Mar 8, 2007 | Reddy Pingili Krishna | A novel amorphous form of linezolid |
| Citing Patent | Filing date | Publication date | Applicant | Title |
|---|---|---|---|---|
| WO2009032294A2 * | Sep 5, 2008 | Mar 12, 2009 | Teva Pharma | Processes for the preparation of a linezolid intermediate, linezolid hydroxide |
| WO2011076678A1 * | Dec 17, 2010 | Jun 30, 2011 | F. Hoffmann-La Roche Ag | Substituted benzamide derivatives |
……………
Filed under: GENERIC DRUG, Uncategorized Tagged: LINEZOLID, ZYVOX












































 COCK WILL TEACH YOU NMR
COCK WILL TEACH YOU NMR COCK SAYS MOM CAN TEACH YOU NMR
COCK SAYS MOM CAN TEACH YOU NMR



 amcrasto@gmail.com
amcrasto@gmail.com