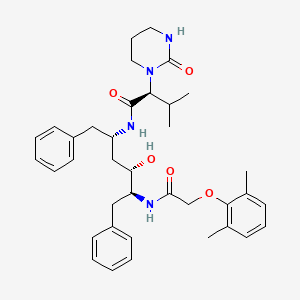
LOPINAVIR
(2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl]-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide
[1S-[1R*,(R*),3R*,4R*]]-N-[4-[[(2,6-dimethyl-phenoxy)acetyl]amino]-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl]tetrahydro-alpha-(1-methylethyl)-2-oxo-1(2H)-pyrimidineacetamide
(2S,3S,5S)-2-(-2,6- dimethylphenoxyacetyl)-amino-3-hydroxy-5-(2-(1-tetrahydropyrimid-2-onyl)-3- methylbutanoyl)amino-1 ,6-diphenylhexane
628.8008
| Abbott Laboratories |
| CAS | 192725-17-0 |
|---|
| AHFS/Drugs.com | International Drug Names |
|---|---|
| MedlinePlus | a602015 |
| Pregnancy cat. | C (US) |
| Legal status | POM (UK) ℞-only (US) |
SYNONYMS
……………
Inhibitors of human immunodeficiency virus (HIV) protease have been approved for use in the treatment of HIV infection for several years. A particularly effective and recently approved HIV protease inhibitor is (2S,3S,5S)-2-(-2,6- dimethylphenoxyacetyl)-amino-3-hydroxy-5-(2-(1-tetrahydropyrimid-2-onyl)-3- methylbutanoyl)amino-1 ,6-diphenylhexane (also known as lopinavir).
Lopinavir
Lopinavir is known to have utility for the inhibition of HIV protease and the inhibition of HIV infection. Lopinavir is particularly effective for the inhibition of HIV protease and for the inhibition of HIV infection when coadministered with ritonavir. Lopinavir, when combined with ritonavir, is also particularly effective for the inhibition of HIV infection when used in combination with one or more reverse transcriptase inhibitors and/or one or more other HIV protease inhibitors.
Lopinavir and processes for its preparation are disclosed in U.S. Patent No. 5,914,332, issued June 22, 1999, which is hereby incorporated herein by reference. This patent also discloses processes for preparing amorphous lopinavir.
Pharmaceutical compositions comprising lopinavir or a pharmaceutically acceptable salt thereof are disclosed in U.S. Patent No. 5,914,332, issued June 22, 1999; U.S. Patent Application No. 08/966,495, filed November 7, 1997; U.S. Provisional Application for Patent No. 60/177,020, filed January 19, 2000 and U.S. Patent Application No. 09/487,739, filed January 19, 2000, all of which are hereby incorporated herein by reference.
Lopinavir (ABT-378) is an antiretroviral of the protease inhibitor class. It is used against HIV infections as a fixed-dose combination with another protease inhibitor, ritonavir, under the trade names Kaletra (high-income countries) and Aluvia (low-income countries). It was first approved by the FDA on 15 September 2000.[1]
Lopinavir (ABT-378) is an antiretroviral of the protease inhibitor class. It is marketed by Abbott as Kaletra, a co-formulation with a sub-therapeutic dose of ritonavir, as a component of combination therapy to treat HIV/AIDS.
Retroviruses are those viruses which utilize a ribonucleic acid (RNA) intermediate and a RNA-dependent deoxyribonucleic acid (DNA) polymerase, reverse transcriptase, during their life cycle. Retroviruses include, but are not limited to, the RNA viruses of the Retroviridae family, and also the DNA viruses of the Hepadnavirus and Caulimovirus families. Retroviruses cause a variety of disease states in man, animals and plants. Some of the more important retroviruses from a pathological standpoint include human immunodeficiency viruses (HIV-1 and HIV-2), which cause acquired immune deficiency syndrome (AIDS) in man, human T-cell lymphotrophic viruses I, II, IV and V, which cause human acute cell leukemia, and bovine and feline leukemia viruses which cause leukemia in domestic animals.
Proteases are enzymes which cleave proteins at specific peptide bonds. Many biological functions are controlled or mediated by proteases and their complementary protease inhibitors. For example, the protease renin cleaves the peptide angiotensinogen to produce the peptide angiotensin I. Angiotensin I is further cleaved by the protease angiotensin converting enzyme (ACE) to form the hypotensive peptide angiotensin II. Inhibitors of renin and ACE are known to reduce high blood pressure in vivo. An inhibitor of a retroviral protease will provide a therapeutic agent for diseases caused by the retrovirus.
The genomes of retroviruses encode a protease that is responsible for the proteolytic processing of one or more polyprotein precursors such as the pol and gag gene products. See Wellink, Arch. Virol. 981 (1988). Retroviral proteases most commonly process the gag precursor into core proteins, and also process the pol precursor into reverse transciptase and retroviral protease. In addition, retroviral proteases are sequence specific. See Pearl, Nature 328 482 (1987).
The correct processing of the precursor polyproteins by the retroviral protease is necessary for the assembly of infectious virions. It has been shown that in vitro mutagenesis that produces protease-defective virus leads to the production of immature core forms which lack infectivity. See Crawford, J. Virol. 53 899 (1985); Katoh, et al., Virology 145 280 (1985). Therefore, retroviral protease inhibition provides an attractive target for antiviral therapy. See Mitsuya, Nature 325 775 (1987).
Current treatments for viral diseases usually involve administration of compounds that inhibit viral DNA synthesis. Current treatments for AIDS involve administration of compounds such as 3′-azido-3′-deoxythymidine (AZT), 2′,3′-dideoxycytidine (DDC), 2′,3′-dideoxyinosine (DDI), d4T and 3TC and compounds which treat the opportunistic infections caused by the immunosuppression resulting from HIV infection. None of the current AIDS treatments have proven to be totally effective in treating and/or reversing the disease. In addition, many of the compounds currently used to treat AIDS cause adverse side effects including low platelet count, renal toxicity and bone marrow cytopenia.
Recently the HIV protease inhibitors ritonavir, saquinavir and indinavir have been approved in the U.S. for treatment of HIV infections. However, there is a continuing need for improved HIV protease inhibitors.
Pharmacology
Lopinavir is highly bound to plasma proteins (98–99%).[2]
Reports are contradictory regarding lopinavir penetration into the cerebrospinal fluid (CSF). Anecdotal reports state that lopinavir cannot be detected in the CSF; however, a study of paired CSF-plasma samples from 26 patients receiving lopinavir/ritonavir found lopinavir CSF levels above the IC50 in 77% of samples.[3]
Clinical properties
Side effects, interactions, and contraindications have only been evaluated in the drug combination lopinavir/ritonavir.
Research
A 2014 study indicates that lopinavir is effective against the
human papilloma virus (HPV). The study used the equivalent of one tablet twice a day applied topically to the cervixes of women with high grade and low grade pre-cancerous conditions. After three months of treatment, 82.6% of the women who had high-grade disease had normal cervical conditions, confirmed by smears and biopsies.[4]
Lopinavir of Formula I is chemically [1S-[1R*,(R*),3R*,4R*]]-N-[4-[[(2,6-dimethyl-phenoxy)acetyl]amino]-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl]tetrahydro-alpha-(1-methylethyl)-2-oxo-1(2H)-pyrimidineacetamide and is indicated in combination with other antiretroviral agents for the treatment of HIV-infection.
U.S. Pat. No. 5,914,332 provides a process for preparing amorphous lopinavir which involves dissolving lopinavir in an organic solvent (for example, ethanol, isopropanol, acetone, or acetonitrile) and then adding the solution to water. For example, lopinavir is dissolved in ethanol (from about 2 to about 4 mL/g) and the ethanolic solution is added with stirring to water (from about 10 about 100 mL/g) to provide amorphous lopinavir. However, this process for the preparation of amorphous lopinavir is not effective on the kilogram scale and thus is not commercially suitable.
PCT Publication No. WO 01/074787 provides various crystalline Forms (Types I, II, III, IV) of solvated and non-solvated lopinavir. It further provides a process for the preparation of amorphous lopinavir which involves dehydration/desolvation of Type I hydrated crystal form/Type II solvated crystal forms.
PCT Publication Nos WO 2006/100552 and WO 2006/090264 provide process for the preparation of crystalline lopinavir.
Organic Process Research & Development, 3, 145-148 (1999), and Organic Process Research & Development, 4, 264-269 (2000); provide a crystallization process for the preparation of crystalline lopinavir which involves recrystallization from mixtures of ethyl acetate and heptane. However, the crystalline lopinavir obtained contains small amounts of solvents and removal of the final traces of solvents proved exceedingly difficult, and even extensive drying after milling (to reduce particle size) did not facilitate its complete removal. It further provides the crystallized product obtained contains appromixately 2% residual ethyl acetate which cannot be removed by further drying.

……………………………….
https://www.google.com/patents/EP0882024A1?dq=5914332&ei=HkCVU9egNtOcugTls4HgDA
Scheme 1
3
Scheme I1A
\
Scheme MB
OH R2 O Scheme III
Scheme IV
10
………………………………..
http://www.google.com/patents/US20110224435
AMORPHOUS FORM
………………………………………………
http://www.google.com.ar/patents/WO2001074787A2?cl=en
POLYMORPHS
……………….
http://www.google.com.ar/patents/US8445506
EXAMPLESExample 1
Thionyl chloride (18 ml) was added to the mixture of 2S-(1-tetrahydropyrimid-2-onyl)-3-methylbutanoic acid (25 gm), tetrahydrofuran (370 ml) and dimethylformamide (2 ml) at 0-10 deg C. and the mass was stirred for 1 hour 15 minutes. The mass was subjected to distillation under reduced pressure to remove excess thionyl chloride, n-heptane (45 ml) was added to the residue obtained and the solvent was distilled off. The reaction mass was slurried in dimethylformamide (105 ml). (2S,3S,5S)-2-(2,6-dimethylphenoxyacetyl)amino-3-hydroxy-5-amino-1,6-diphenylhexane (41 gm), imidazole (25 gm) and 4-(dimethylamino)pyridine (1.5 gm) were dissolved in ethyl acetate (420 ml). To the solution was added above slurried product at 0-10 deg C. The reaction mass was maintained for 14 hours and then ethyl acetate (165 ml) and water (250 ml) were added. The layers were separated, water (250 ml) was added to the organic layer and the pH was adjusted to 2.0-3.0 with dilute hydrochloric acid (6N HCl). The layers were separated, the organic layer was washed with aqueous sodium bicarbonate and then with water. The ethyl acetate was distilled off from the mass. The reaction mass was dissolved in ethyl acetate (80 ml) and n-heptane (80 ml) was added to the solution. The separated solid was stirred with ethyl acetate (290 ml) for 8 hours, filtered and dried the solid to obtain 33 gm of lopinavir ethyl acetate solvate
……………………………
http://www.google.com/patents/US20130267547

………………………………….
http://pubs.acs.org/doi/abs/10.1021/op990202j
A large scale process for the synthesis of HIV protease inhibitor candidate ABT-378 has been developed which utilizes an intermediate common to the synthesis of ritonavir, Abbott’s first generation compound. The synthesis relies on the sequential acylation of this intermediate which is carried through as a mixture of diastereomers until the penultimate step. A synthesis of acid 5, derived from l-valine, is also reported.
-
Crystallographic studies have shown, to our surprise, that 2 isolated by this crystallization method is not a solvate.
-
The determination of the enantiomeric excess (% ee) for ABT-378 (2) can be done indirectly. Compound 17, which results from the acylation of 4 with the enantiomer of acid 5, is known to us, having been detected as an impurity in our process development.17 Compound 18 can only result from the acylation of the enantiomer of 4 (2R,3R,5R) with 5. The levels of 17/18 observed in 2 are typically <0.1%. Until there is a need for a more definitive assay, we assume this represents an upper limit to the amount of ent-2 present.
References
- “FDA Approved Drug Products: Kaletra”. Retrieved 30 April 2004.
- KALETRA (lopinavir/ritonavir) capsules; (lopinavir/ritonavir) oral solution. Prescribing information. April 2009
- Capparelli E, Holland D, Okamoto C, et al. (2005). “Lopinavir concentrations in cerebrospinal fluid exceed the 50% inhibitory concentration for HIV”. AIDS (London, England) 19 (9).
- HIV drug used to reverse effects of virus that causes cervical cancer University of Manchester, 17 February 2014.
|
8-20-2003
|
Crystalline pharmaceutical
|
|
|
12-27-2002
|
Compositions and methods for enhancing the bioavailability of pharmaceutical agents
|
|
|
10-13-2000
|
PREGELATINIZED STARCH IN A CONTROLLED RELEASE FORMULATION
|
|
|
6-20-1997
|
RETROVIRAL PROTEASE INHIBITING COMPOUNDS
|
|
8-8-2012
|
PROCESS FOR THE PREPARATION OF SUBSTANTIALLY PURE (2S,3S,5S)-5-AMINO-2-N,N-DIBENZYLAMINO-3-HYDROXY-1,6-DIPHENYLHEXANE
|
|
|
11-12-2010
|
PRODRUGS OF HIV PROTEASE INHIBITORS
|
|
|
5-19-2010
|
Prodrugs of HIV protease inhibitors
|
|
|
5-7-2010
|
DIMETHYLPHENOXY MODULATORS OF VIRAL PROTEASE ACTIVITY AND/OR PARASITIC ENZYME ACTIVITY
|
|
|
1-12-2007
|
Methods of treating cancer
|
|
|
9-21-2005
|
Method to design therapeutically important compounds
|
|
|
6-10-2005
|
Crystalline pharmaceutical
|
|
|
3-9-2005
|
Crystalline pharmaceutical
|
|
|
2-4-2005
|
Methods and compositions for the treatment or prevention of human immunodeficiency virus and related conditions using cyclooxygenase-2 selective inhibitors and antiviral agents
|
|
|
8-27-2004
|
Methods of treating cancer
|
Filed under: Uncategorized Tagged: LOPINAVIR