Image may be NSFW.
Clik here to view.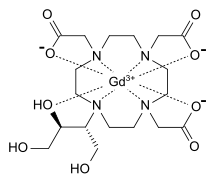 GADOBUTROL
GADOBUTROL
gadolinium(III) 2,2′,2”-(10-((2R,3S)-1,3,4-trihydroxybutan-2-yl)-1,4,7,10-tetraazacyclododecane-1,4,7-triyl)triacetate
Gadobutrol, SH-L-562, Gadovist,138071-82-6
The US Food and Drug Administration (FDA) has approved Bayer HealthCare’s Gadavist (gadobutrol) injection as the first magnetic resonance contrast agent for evaluation of breast cancer in the US.
The agency has approved the new indication for Gadavist injection for intravenous use with magnetic resonance imaging of the breast to assess the presence and extent of malignant breast disease.
Approval is based on priority review of two Phase III studies with identical design (GEMMA-1 and GEMMA-2).
Bayer HealthCare’s Gadavist (gadobutrol)
Bayer’s Gadavist injection cleared for breast cancer evaluation
The US Food and Drug Administration (FDA) has approved Bayer HealthCare’s Gadavist (gadobutrol) injection as the first magnetic resonance contrast agent for evaluation of breast cancer in the US.
Image may be NSFW.
Clik here to view. GADOBUTROL
GADOBUTROL
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Licence data | US FDA:link |
| Pregnancy cat. | C (US) |
| Legal status | POM (UK) ℞-only (US) |
| Routes | IV |
| Identifiers | |
| CAS number | 138071-82-6 Image may be NSFW. Clik here to view.  |
| ATC code | V08CA09 |
| PubChem | CID 72057 |
| DrugBank | DB06703 |
| UNII | 1BJ477IO2L Image may be NSFW. Clik here to view.  |
| KEGG | D07420 Image may be NSFW. Clik here to view.  |
| Chemical data | |
| Formula | C18H31GdN4O9 |
| Mol. mass | 604.710 g/mol |
………………………..
Gadobutrol (INN) (Gd-DO3A-butrol) is a gadolinium-based MRI contrast agent (GBCA).
It received marketing approval in Canada[1] and in the United States.[2][3][4]
As of 2007, it was the only GBCA approved at 1.0 molar concentrations.[5]
Gadobutrol is marketed by Bayer Schering Pharma as Gadovist, and by Bayer HealthCare Pharmaceuticals as Gadavist.[6]
Image may be NSFW.
Clik here to view.
Image may be NSFW.
Clik here to view.
Image may be NSFW.
Clik here to view. WORLDCUP FOOTBALL WEEK 2014 BRAZIL
WORLDCUP FOOTBALL WEEK 2014 BRAZIL
……………………………………………….
http://www.google.com/patents/EP0988294B1?cl=en
-
This type of complexes with metal ions, in particular with paramagnetic metal ions; is used for the preparation of non-ionic contrast agents for the diagnostic technique known as magnetic resonance (MRI, Magnetic Resonance Imaging), among which are ProHance(R) (Gadoteridol, gadolinium complex of 10-(2-hydroxypropyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triacetic acid), and Gadobutrol (gadolinium complex of [10-[2,3-dihydroxy-1-(hydroxymethyl)propyl]-1,4,7,10-tetraazacyclododecane-1,4,7-triacetic acid).
-
[0003]Two different synthetic approaches are described in literature for the preparation of this kind of complexes, said approaches differing in the strategy taken to discriminate one of the four nitrogen atoms: the first one (Dischino et al., Inorg. Chem., 1991, 30, 1265 or EP 448191, EP 292689, EP 255471) is based on the selective protection of one of the nitrogen atoms by formation of the compound of formula (III), 5H,9bH-2a,4a,7-tetraazacycloocta[cd]pentalene, and on the subsequent hydrolysis to compound of formula (IV), 1-formyl-1,4,7,10-tetraazacyclododecane, followed by the carboxymethylation of the still free nitrogen atoms and by the deprotection and alkylation of the fourth nitrogen atom, according to scheme 1.
-
[0004]The step from 1,4,7,10-tetraazacyclododecane disulfate (a commercially available product) to compound (III) is effected according to the conventional method disclosed in US 4,085,106, followed by formation of the compound of formula (IV) in water-alcohol medium.
-
[0005]This intermediate is subsequently tricarboxymethylated with tert-butyl bromoacetate (TBBA) in dimethylformamide at 2.5°C and then treated with a toluene-sodium hydroxide diphasic mixture to give the compound of formula (V), 10-formyl-1,4,7,10-tetraazacyclododecane-1,4,7-triacetic, tris(1,1-dimethylethyl) ester, which is subsequently hydrolysed to compound of formula (II) in acidic solution.
-
[0006]In the process described in WO 93/24469 for the synthesis of Gadobutrol, at first one of the nitrogen atoms is alkylated in conditions such as to minimize the formation of polyalkylated derivatives, then the monoalkylderivative is purified and carboxymethylated, according to scheme 2.
-
[0007]The alkylation of 1,4,7,1,0-tetraazacyclododecane with the epoxide of formula (VI), 4,4-dimethyl-3,5,8-trioxabicyclo[5.1.0]octane, is carried out in anhydrous n-BuOH under reflux and the reaction mixture is extracted with water, evaporated to dryness and the residue is subsequently diluted with water and extracted with methylene chloride.
-
[0008]The aqueous phase containing the mono-alkylated product (65% yield in Example 7 which reports the procedure for the preparation of 5 kg of Gadobutrol) is directly carboxymethylated at 70°C with chloroacetic acid, keeping pH 9.5 by addition of NaOH. The reaction mixture is adjusted to pH 1, concentrated to dryness and dissolved in methanol to remove the undissolved salts. The filtrate is then concentrated under vacuum, dissolved in water, and loaded onto a cation exchanger in the H+ form to fix the product. The subsequent elution with ammonia displaces the desired product, which is concentrated to small volume and subsequently complexed with gadolinium oxide according to conventional methods, and the resulting complex is purified by means of ion exchange resins. The overall yield is 42%.
-
[0009]Although the first of these two processes could theoretically provide a higher yield, in that all the single steps (protection, carboxymethylation and deprotection) are highly selective, the complexity of the operations required to remove salts and solvents and to purify the reaction intermediates makes such theoretical advantage ineffective: the overall yield is in fact, in the case of Gadoteridol, slightly higher than 37%.
-
[0010]The preparation of Gadobutrol according to the alternative process (WO 93/24469) provides a markedly better yield (72%) only on laboratory scale (example 2): example 7 (represented in the above Scheme 2) actually evidences that, when scaling-up, the yield of this process also remarkably decreases (42%).
-
[0011]In addition to the drawback of an about 40% yield, both processes of the prior art are characterized by troublesome operations, which often involve the handling of solids, the use of remarkable amounts of a number of different solvents, some of them having undesirable toxicological or anyway hazardous characteristics.
-
[0012]Moreover, the synthesis described by Dischino makes use of reagents which are extremely toxic, such as tert-butyl bromoacetate, or harmful and dangerous from the reactivity point of view, such as dimethylformamide dimethylacetal.
-
[0013]An alternative to the use of dimethyl formamide dimethylacetal is suggested by J. Am. Chem. Soc. 102(20), 6365-6369 (1980), which discloses the preparation of orthoamides by means of triethyl orthoformate.
-
[0014]EP 0596 586 discloses a process for the preparation of substituted tetraazacyclododecanes, among them compounds of formula (XII), comprising:
- formation of the tricyclo[5.5.1.0] ring;
- alkylation with an epoxide;
- hydrolysis of the 10-formyl substituent;
- reaction with an acetoxy derivative bearing a leaving group at the alpha-position.
-
[0015]Nevertheless, this method requires quite a laborious procedure in order to isolate the product of step b).
-
[0016]It is the object of the present invention a process for the preparation of the complexes of general formula (XII)
wherein
- R1 and R2
- are independently a hydrogen atom, a (C1-C20) alkyl containing 1 to 10 oxygen atoms, or a phenyl, phenyloxy group, which can be unsubstituted or substituted with a (C1-C5) alkyl or hydroxy, (C1-C5) alkoxy, carbamoyl or carboxylic groups,
- Me3+
- is the trivalent ion of a paramagnetic metal;
comprising the steps represented in the following Scheme 3:
-
The process of the present invention keeps the high selectivity typical of the protection/deprotection strategy described by Dischino in the above mentioned paper, while removing all its drawbacks, thus providing for the first time a reproducible industrial process for the preparation of the concerned compounds in high yields and without use of hazardous substances.
-
[0019]The preparation of the gadolinium complex of 10-(2-hydroxypropyl)-1,4,7,10-tetraazacyclododecane-1,4,7-tri-acetic) acid (Gadoteridol), according to scheme 4, is particularly preferred:
in which the synthetic steps a), b), c), d), e), and f) have the meanings defined above and the epoxide of formula (XI) in step d) is propylene oxide.
-
[0020]The preparation of the gadolinium complex of [10-[2,3-dihydroxy-1-(hydroxymethyl)propyl]-1,4,7,10-tetraazacyclododecane-1,4,7-triacetic) acid (Gadobutrol), according to the scheme 5, is also preferred.
in which the synthetic steps a), b), c), d), e), and f) have the meanings defined above and the epoxide of formula (XI) in step d) corresponds to the one of formula (VI), defined above.
-
[0021]On the other hand, step a) of the process of the present invention involves the use of triethyl orthoformate in the presence of an acid catalyst, instead of dialkylformamide-dialkylacetal.
-
[0022]Triethyl orthoformate can be added in amounts ranging from 105% to 200% on the stoichiometric value.
-
[0023]The reaction temperature can range from 110 to 150°C and the reaction time from 5 to 24 h.
-
[0024]The catalyst is a carboxylic acid having at least 3 carbon atoms, C3-C18, preferably selected from the group consisting of propionic, butyric and pivalic acids.
-
[0025]Triethyl orthoformate is a less toxic and less expensive product than N,N-dimethylformamide-dimethylacetal and does not involve the formation of harmful, not-condensable gaseous by-products. Moreover, triethyl orthoformate is less reactive than N,N-dimethylformamide-dimethylacetal, which makes it possible to carry out the loading procedures of the reactives as well as the reaction itself in utterly safe conditions even on a large scale, allows to better monitor the progress of the reaction on the basis of such operative parameters as time and temperature, without checking the progress by gas chromatography, and makes dosing the reactive less critical, in that it can be added from the very beginning without causing the formation of undesired by-products: all that rendering the process suitable for the production of compound (III) on the industrial scale in easily reproducible conditions.
-
[0026]The subsequent step b) involves the carboxymethylation of compound (III) in aqueous solution, using a haloacetic acid, to give compound (IX), i.e. the 10-formyl-1,4,7,10-tetraazacyclododecane-1,4,7-triacetic acid salt with an alkali or alkaline-earth metal, the salts of compound (IX) with sodium, potassium or calcium being most preferred.
Example 2
-
[0065]
-
[0066]The procedure of Example 1 is followed until step C included, to obtain a solution of DO3A trisodium salt.
-
[0067]pH is adjusted to 12.3 with conc. HCl and 57.7 kg (0.4 kmol) of 4,4-dimethyl-3,5,8-trioxabicyclo[5.1.0]-octane are added. After reaction for 4 h at 40°C and for 8 h at 80°C, the solution is cooled to 50°C, 120 kg of an aqueous solution containing 0.135 kmol of gadolinium trichloride are added. After 1 h the mixture is cooled at 17°C and acidified to pH 1.7 with conc. HCl, keeping this pH for 2 h. The solution is subsequently warmed to 50°C, pH is adjusted to 7 with sodium hydroxide, keeping these conditions for 1 h.
-
[0068]After that, the resulting crude Gadobutrol is purified repeating exactly the same process as in steps E and F of Example 1.
Recovery of the product (Gadobutrol)
-
[0069]The product-rich fraction is then thermally concentrated to a viscous residue and the residue is added with 350 kg of ethanol at 79°C.
-
[0070]The resulting suspension is refluxed for 1 h, then cooled, centrifuged and dried under reduced pressure to obtain 66.0 kg of Gadobutrol (0.109 kmol), HPLC assay 99.5% (A%).
Overall yield: 79.1% -
[0071]The IR and MS spectra are consistent with the indicated structure.
Image may be NSFW.
Clik here to view.
References
- Cheng, KT (2007). “Gadobutrol”. Molecular Imaging and Contrast Agent Database (MICAD) (Bethesda, MD: National Center for Biotechnology Information (NCBI)). PMID 20641787. NBK23589.
- http://bayerimaging.com/products/gadavist/index.php
- “FDA approves imaging agent for central nervous system scans” (Press release). U.S. Food and Drug Administration (FDA). March 15, 2011. Retrieved March 31, 2011.
- “U.S. FDA Approves Bayer’s Gadavist (Gadobutrol) Injection for MRI of the Central Nervous System” (Press release). Bayer HealthCare Pharmaceuticals. March 14, 2011. Retrieved March 31, 2011.
- “Gadobutrol 1.0-molar in Cardiac Magnetic Resonance Imaging (MRI) – Further Enhancing the Capabilities of Contrast-enhanced MRI in Ischaemic and Non-ischaemic Heart Disease?”
- “Gadavist full prescribing information”. Retrieved 2011-03-14.Image may be NSFW.
Clik here to view.
Image may be NSFW.
Clik here to view.
Filed under: cancer, Contrast agent, FDA 2014 Tagged: Bayer HealthCare's, breast cancer, fda, Gadavist, gadobutrol Image may be NSFW.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.









