
By Frederic L. “Rick” Sax, M.D., global head for the Center for Integrated Drug Development, Quintiles.
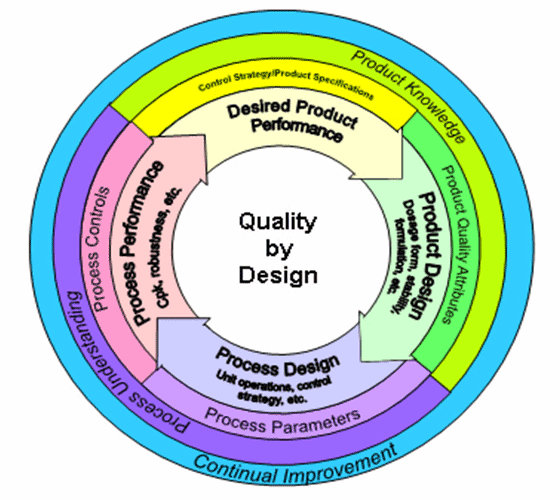
The biopharmaceutical manufacturing industry has used quality by design (QbD) principles for decades. The essence of QbD is designing with the end in mind (in this case, the efficient manufacture of a high-quality drug product). This approach emphasizes that the operative word in QbD is not quality, but design.
read all at
http://www.pharmaceuticalonline.com/doc/how-to-apply-qbd-principles-in-clinical-trials-0001


\
Filed under: CLINICAL TRIALS, Regulatory Tagged: Center for Integrated Drug Development, clinical trials, Frederic L., QbD, QbD Principles, quality by design, Quintiles