 |
|
| Systematic (IUPAC) name | |
|---|---|
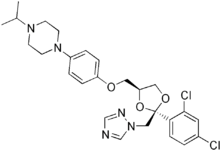
| 1-[4-[ [(2S,4S)-2-(2,4-Dichlorophenyl)-2- (1,2,4-triazol-1-ylmethyl)- 1,3-dioxolan-4-yl]methoxy]phenyl]- 4-propan-2-yl-piperazine | |
| Clinical data | |
| Trade names | Terazol |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a688022 |
| Legal status | ? |
| Pharmacokinetic data | |
| Protein binding | 94.9% |
| Identifiers | |
| CAS number | 67915-31-5  |
| ATC code | G01AG02 |
| PubChem | CID 441383 |
| DrugBank | DB00251 |
| ChemSpider | 390122  |
| UNII | 0KJ2VE664U  |
| KEGG | D00888  |
| ChEMBL | CHEMBL1306  |
| Chemical data | |
| Formula | C26H31Cl2N5O3 |
| Mol. mass | 532.462 g/mol |

Terconazole is an anti-fungal medication, primarily used to treat vaginal fungal infections.
The synthesis of racemic terconazole [J. Heeres et al., J. Med . Chem . , 26 , 611 11983)] is similar. differing in the introduction of a 1 H- 1 , 2,4-triazol-1-yl substituent in place of 1H-imidazol-1-yl and in the nature of the phenol used in the last step of the synthetic sequence, which phenol is 1-methylethyl-4-(4- hydroxyphenyl)piperazme instead of 1-acetyl-4-(4-nydroxyphenyl)piperazine.
Example 20: (2S,4R) -(-)-1-[4-[[2-(2,4-dichlorophenyl)-2-[(1H-1,2,4-triazol-1-yl]methyl-1,3-dioxolane-4-yl]methoxy]phenyl]-4-(1-methylethyl)piperazine, (2S,4R) – (-)-terconazole.
This compound is prepared following the process described for (+)-torconazole, starting from (2S,4S)-(-)-IV (Ar = 2,4-dichlorophenyl, Y = N, R = CH3) (224 mg, 0.55 mmol), 4-(4-hydroxyphenyl)-1-(1-methylethyl)-piperazine (121 mg, 0.55 mmol), NaH (22.4 mg, 0.56 mmol) in 8 ml of DMSO. (2S,4R) -(-(-terconazole ((2S,4R)-V, Ar
= 2,4-dichlorophenyl, Y = N, Z = CH(CH3)2) is obtained as a white solid, m.p. 76-78ºC, [α]D 20= -12.0 (c = 0.4.
CHCl3).
Example 17 : (2R,4S)-(+)-1-[4-[[2-(2,4-dichlorophenyl)- 2-[(1H-1,2,4-triazol-1-yl]methyl-1,3-dioxolane-4-yl]methyl]phenyl]-4-(1-methylethyl)piperazine, (2R,4S)-(+)-terconazole.
To a suspension of NaH (60-65% dispersion in paraffin, 36 mg, 0.90 mmol) in anhydrous DMSO (8 ml), 4-(4-hydroxyphenyl) -1 – ( 1-methyle thyl ) p iper az ine ( 193 mg , 0 . 88 mmol ) is added and the mixture is stirred for 1 hour at room temperature. Then, (2R,4R)-(+)-IV (Ar = 2,4-dichlorophenyl, Y = N, R = CH3 ) is added (180 mg, 0.44 mmol) and the mixture is heated at 80°C for 4 hours. The reaction mixture is allowed to cool to room temperature, diluted with water (20 ml) and extraoteo with CH2Cl2 (3 × 25 ml). The combined organic phases are washed with 5N NaOH (3 × 25 ml) and water (3 × 25 ml dried with Na2SO4 and the solvent is evaporated of: under vacuum. The oily residue thus obtained is crystallized from diisopropyl ether to give (2R,4S)-(+)-terconazole ((2R,4S)-V, Ar = 2,4-cichlorophenyl, Y = N, Z = CH(CH3)2) (140 mg, 59 % yield) as a white solid, m.p. 72-74’C, [α]D 20 = + 11,05 (c = 0.4, CHCl3).
IR (KBr), ʋ : 1585, 1512, 1454, 1380, 1270, 1239, 1137, 1048, 979, 820, 675 cm-1.
1H-NMR (200 MHz, CDCl3), δ : 1.11 [d, J=6.5 Hz, 5H, (CH3)2CH], 2.73 [m, 5H, 3-H2, 5-H2 and (CH3)2CH], 3.49
(dd, J=9.6 Hz, J’=6.3 Hz, 1H), 3.80 (m, 2H ) and 3.91
(dd, J=8.2 Hz, J’=6.6 Hz, 1H) (4′ ‘-CH2 and 5′ ‘-H2), 4.35
(m, 1H, 4′ ‘-H), 4.74 (d, J=14.6 Hz, 1H) and 4.84 (d, J=14.6 Hz, 1H) (CH2-N), 6.76 [d, J=9.0 Hz, 2H, C2'(6')- H], 6.88 [d, J=9.0 Hz, 2H, C3'(5')-H], 7.24 (dd, J=8.5
Hz, J’=2.0 Hz, 1H, 5”’-H), 7.46 (d, J=2.0 Hz, 1H,
3″‘-H), 7.56 (d, J=8.5 Hz, 1H, 6″‘-H), 7.89 (s, 1 H) and
8.20 (s, 1H) (triazole 3-H and 5-H).

Synthesis pathway
![]()
-
DE 2804096 (Janssen; appl. 3.8.1978; prior. 31.1.1978).
-
US 4,358,449 (Janssen; 9.11.1982; prior. 21.11.1977).
-
US 4,144,346 (Janssen; 13.3.1979; prior. 21.11.1977, 31.1.1977).
-
US 4,223,036 (Janssen; 16.9.1980; prior. 8.1.1979, 21.11.1977, 31.1.1977).
-
Heeres, J. et al .: J. Med. Chem. (JMCMAR) 26, 611 (1983).
Filed under: Uncategorized Tagged: TERCONAZOLE






