![]()
ICOTINIB
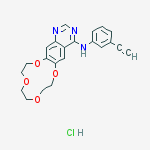
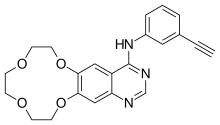
4-((3-ethynylphenyl)amino)-6,7-benzo-12-crown-4-quinazoline
N-(3-Ethynylphenyl)-7,8,10,11,13,14-hexahydro[1,4,7,10]tetraoxacyclododecino[2,3-g]quinazolin-4-amine
[1,4,7,10]Tetraoxacyclododecino[2,3-g]quinazolin-4-amine, N-(3-ethynylphenyl)-7,8,10,11,13,14-hexahydro-
BPI 2009H, UNII-JTD32I0J83
610798-31-7 CAS BASE
![Compound Structure]()
Icotinib Hydrochloride, 1204313-51-8, CS-0918, HY-15164, Conmana Zhejiang Beta Pharma Ltd.
CLINICALS………http://clinicaltrials.gov/search/intervention=Icotinib
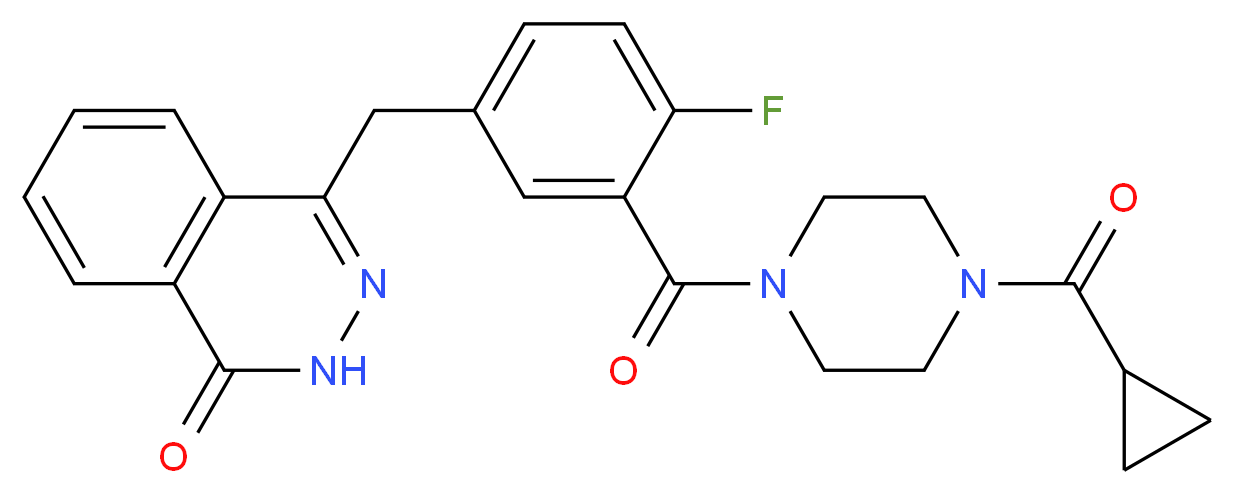
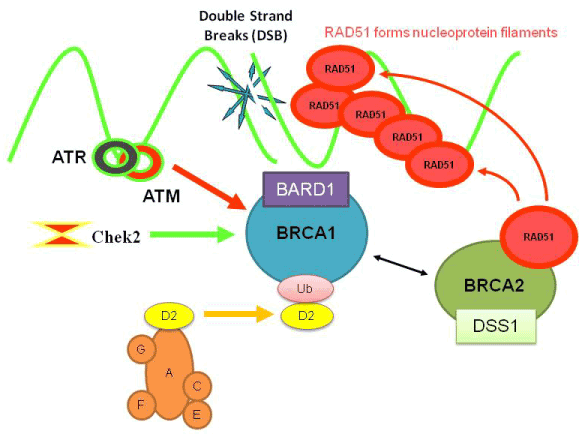
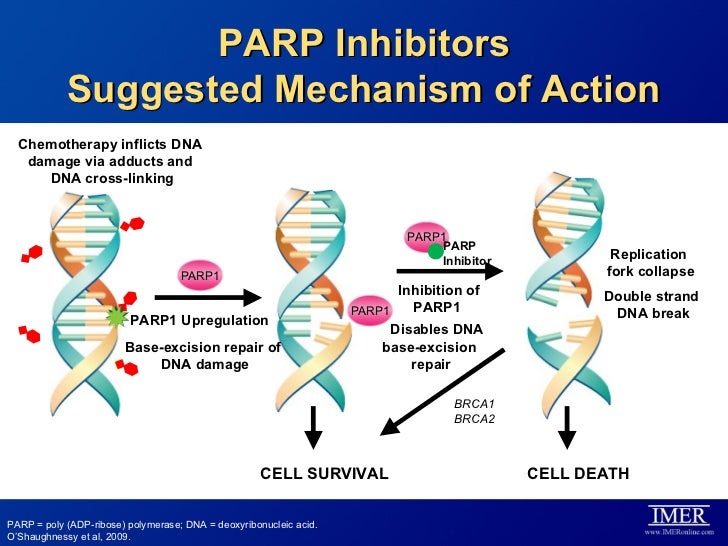
Icotinib Hydrochloride (BPI-2009H), or Icotinib, is a highly selective, first generation epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI). EGFR is an oncogenic driver and patients with somatic mutations, particularly an exon 19 deletion or exon 21 L858R mutation, within the tyrosine kinase domain have activating mutations that lead to unchecked cell proliferation. Overexpression of EGFR causes inappropriate activation of the anti-apoptotic Ras signaling pathway, found in many different types of cancer. Icotinib is a quinazoline derivative that binds reversibly to the ATP binding site of the EGFR protein, preventing completion of the signal transduction cascade.[1]
Clinical Evaluation
Icotinib is indicated for the treatment for EGFR mutation-positive, advanced or metastatic non-small cell lung cancer (NSCLC) as a second-line or third-line treatment, for patients who have failed at least one prior treatment with platinum-based chemotherapy. The ICOGEN trial was a double-blind, head-to-head phase III study comparing icotinib with gefitinib in all-comers. From 27 centers in China, 399 patients were randomized between the two treatments testing for a primary objective of progression-free survival and secondary objectives of overall survival, time to progression, quality of life, percentage of patients who achieved an objective response, and toxic effects. The ICOGEN results showed icotinib to have a median PFS of 4.6 months (95% CI 3.5 – 6.3) as compared to gefitinib which has a PFS of 3.4 months (95% CI 2.3 – 3.8). After the study was completed, post-hoc analysis revealed that in the icotinib treatment group, patients with activating EGFR mutations showed improved PFS as compared to patients with wild-type EGFR. Icotinib also was associated with fewer adverse events than gefitinib when considering all grades of reactions together (61% versus 70% respectively, p = 0.046).[2] The phase IV ISAFE trial evaluated 5,549 patients and showed icotinib to have an overall response rate of 30% and a low adverse event rate of 31.5%.[3]
Regulatory Approvals
Icotinib was approved in China by the SFDA in June, 2011.[4] Since approval, Icotinib has treated over 40,000 patients in China successfully and is now undergoing global development.
January 2014, Beta Pharma, Inc. was given a “May Proceed” from the US FDA to conduct a Phase I study for the evaluation of icotinib as a treatment of EGFR+ Non-Small Cell Lung Cancer (NSCLC).
Icotinib is a potent and specific EGFR inhibitor with IC50 of 5 nM, including the EGFR, EGFR(L858R), EGFR(L861Q), EGFR(T790M) and EGFR(T790M, L858R). Phase 4.Icotinib hydrochloride is the epidermal growth factor receptor kinase targeting a new generation of targeted anti-cancer drugs, completely independent from the original tumor clinical practitioners and experts of science, through eight years of the development, its first adaptation disease is advanced non-small cell lung cancer. Icotinib is an orally available quinazoline-based inhibitor of epidermal growth factor receptor (EGFR), with potential antineoplastic activity. Icotinib selectively inhibits the wild-type and several mutated forms of EGFR tyrosine kinase. This may lead to an inhibition of EGFR-mediated signal transduction and may inhibit cancer cell proliferation. EGFR, a receptor tyrosine kinase, is upregulated in a variety of cancer cell types. Icotinib was approved in China in 2011
Icotinib has been found to be noninferior to gefitinib in patients with non-small-cell lung cancer (NSCLC), according to reports from the phase III Chinese double-blind ICOGEN study.
“[I]cotinib is a valid therapeutic option for patients with non-small-cell lung cancer as a second-line or third-line treatment, although patients might find taking icotinib three times a day an inconvenience,” write Yan Sun (Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China) and colleagues.
Icotinib is an oral epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) that has exhibited good antitumor activity in phase II studies. However, it has a shorter half-life than gefitinib, another TKI, which means that it needs to be taken more often.
Design and discovery of 4-anilinoquinazoline ureas as multikinase inhibitors targeting BRAF, VEGFR-2 and EGFR. Qingwen Zhang, Yuanyuan Diao, Fei Wang, Ying Fu, Fei Tang, Qidong You, Houyuan Zhou,
Med. Chem. Commun., 2013,
4, 979
…
-
Tyrosine kinase receptors are trans-membrane proteins that, in response to an extracellular stimulus, propagate a signaling cascade to control cell proliferation, angiogenesis, apoptosis and other important features of cell growth. One class of such receptors, epidermal growth factor receptor (EGFR) tyrosine kinases, are over-expressed in many human cancers, including brain, lung, liver, bladder, breast, head and neck, esophagus, gastrointestinal, breast, ovary, cervix or thyroid cancer.
-
EGFR is expressed in many types of tumor cells. Binding of cognate ligands (including EGF, TGFα (i.e., Transforming Growth Factor-α) and neuregulins) to the extracellular domain causes homo- or heterodimerization between family members; the juxtaposition of cytoplasmic tyrosine kinase domains results in transphosphorylation of specific tyrosine, serine and threonine residues within each cytoplasmic domain. The formed phosphotyrosines act as docking sites for various adaptor molecules and subsequent activation of signal transduction cascades (Ras/mitogen-activated, PI3K/Akt and Jak/STAT) that trigger proliferative cellular responses.
-
Various molecular and cellular biology and clinical studies have demonstrated that EGFR tyrosine kinase inhibitors can block cancer cell proliferation, metastasis and other EGFR-related signal transduction responses to achieve clinical anti-tumor therapeutic effects. Two oral EGFR kinase inhibitors with similar chemical structures are Gefitinib (Iressa; AstraZeneca), approved by the U.S. FDA for advanced non-small cell lung cancer in 2003 (and later withdrawn), and Erlotinib Hydrochloride (Tarceva; Roche and OSI), approved by the U.S. FDA for advanced non-small cell lung cancer and pancreatic cancer treatment in 2004.
-
Chinese Patent Publication No.
CN1305860C discloses the structure of 4-[(3-ethynyl-phenyl)amino]-6,7-benzo-12-crown-quinoline (free base) on page 29, Example 15, Compound 23.
Icotinib was launched in China in August 2011, after approval by the State Food and Drug Administration. It is a targeted EGFR tyrosine kinase inhibitor that, like erlotinib (Tarceva) and gefitinib (Iressa), shows benefit in patients with EGFR m+ NSCLC.
……………………………………..
http://www.google.com/patents/EP2392576A1
![Figure imgb0011]()
Method 1:
Method 2:
Method 3:
http://www.google.com/patents/EP2392576A1 Example 1Step 1
-
-
Preparation: 16 kg (400 mol) of sodium hydroxide was dissolved in 80 L of water in a 400 L reactor, and then 18.8 L (140 mol) of triethylene glycol, 32 L of THF were added into the reactor. After cooling below 5 °C, a solution of 47.84 kg (260 mol) of tosyl chloride and 50 L of THF was added dropwise. Following the addition, the reaction mixture was kept at this temperature for 2 hours, and it was then poured into 240 L of ice water. The precipitate was formed and filtered, washed with a small amount of water, and dried. 58.64 kg of BPI-01 as a white crystalline powder was yielded at 91.4%. mp: 77-80 °C, HPLC: 97%. TLC (petroleum ether: ethyl acetate = 1:1) Rf = 0.87.
-
NMR data: 1H-NMR (CDCl3): δ ppm: 7.78 (d, 4H, J = 10.4 Hz, benzene protons by sulfonyl group); 7.34 (d, 4H, J = 11.6 Hz, benzene protons by methyl group); 4.129 (dd, 4H, J = 5.6 Hz, ethylene protons by the sulfonyl group); 3.64 (dd, 4H, J = 5.6 Hz, ethylene protons away from the sulfonyl group); 3.517 (s, 4H, ethylene protons in the middle); 2.438 (s, 6H, methyl protons on the benzene).
Step 2
-
-
Preparation: A solution containing 3.64 kg (20 mol) of ethyl 3,4-dihydroxybenzoate and 12.4 kg (89.6 mol) of potassium carbonate in 300 L of N,N-dimethylformamide was stirred and heated to 85-90 °C for about 30 minutes. A solution of 9.17 kg (20 mol) of BPI-01 in 40 L of N,N-dimethylformamide was added dropwise over 1.5-2 hours. After the addition, the reaction was kept for 30 minutes; the reaction completion was confirmed by TLC (developing solvent: petroleum ether:ethyl acetate = 1:1, Rf = 0.58). The reaction mixture was removed from the reactor and filtered. Then, the filtrate was evaporated to remove N,N-dimethylformamide; 240 L of ethyl acetate was added to dissolve the residue. After filtration and vacuum evaporation, the residual solution was extracted with 300 L of petroleum ether. After evaporation of the petroleum ether, the residual solids were re-crystallized with isopropanol in a ratio of 1:2.5 (W/V); 1.68 kg of BPI-02 as a white powder was obtained in a yield of 28%. mp: 73-76 °C, HPLC: 96.4%. NMR data: 1H-NMR (CDCl3): δ ppm: 7.701 (d, 1H, J = 2.4 Hz, benzene proton at position 6); 7.68 (s, 1 H, benzene proton at position 2); 6.966 (d, 1H, J = 10.8 Hz, benzene proton at position 5); 4.374-3.81 (q, 2H, J = 9.6 Hz, methylene protons of the ethyl); 3.78-4.23 (dd, 12H, J = 4.8 Hz, crown ether protons); 1.394 (t, 3H, J = 9.6 Hz, methyl protons of the ethyl). MS: m/z 296.
Step 3
-
-
Preparation: A solution of 592 g (2 mol) of BPI-02 and 600 mL of acetic acid in a 5 L reaction flask was cooled to 0°C; 1640 mL (25.4 mol) of concentrated nitric acid was slowly added. The internal temperature should not exceed 10 °C. While cooled below 0°C, 1 L of concentrated sulfuric acid was added dropwise. The internal temperature should not be higher than 5°C. After the addition, the reaction was kept at 0-5 °C for 1-2 hours. After completion of the reaction, the reaction solution was poured into 15 L of ice water in a plastic bucket. After mixing, filtration, and re-crystallization in ethanol, 449 g of BPI-03 as a light yellow to yellow crystalline powder was obtained in 65.7% yield. mp: 92-95 °C, HPLC: 98.2%. TLC (petroleum ether: ethyl acetate =1:1) Rf = 0.52. NMR data: 1H-NMR (CDCl3): δ ppm: 7.56 (s, 1H, benzene proton at position 5); 7.20 (s, 1H, benzene proton at position 2); 4.402 (q, 2H, J = 9.2 Hz, methylene protons of the ethyl); 4.294 (dd, 12H, J = 4.8 Hz, crown ether protons); 1.368 (t, 3H, J = 9.2 Hz, methyl protons of the ethyl).
Step 4
-
-
Preparation: In a 3 L hydrogenation reactor, 2 L of methanol and 195 g (0.57 mol) of BPI-03 were added, and then 63 mL of acetyl chloride was slowly added. After a short stir, 33 g of Pd/C containing 40% water was added. The reaction was conducted under 4 ATM hydrogen until hydrogen absorption stopped, and then the reaction was kept for 1-2 hours. After completion of the reaction, the reaction mixture was transferred into a 5 L reactor. After filtration, crystallization, and filtration, the product was obtained. The mother liquor was concentrated under vacuum, and more product was obtained. The combined crops were 168 g of BPI-04 as a white to pink crystalline powder in a yield of 85%. mp: 198-201 °C, HPLC: 99.1 %. TLC (petroleum ether: ethyl acetate = 1:1) Rf = 0.33. NMR data: 1H-NMR (DMSO-d6): δ ppm: 8-9 (br., 3H, 2 protons of the amino group and a proton of the hydrochloric acid); 7.37 (s, 1H, benzene proton at position 5); 6.55 (s, 1H , benzene proton at position 2); 4.25 (q, 2H, J = 7.06 Hz, methylene protons of the ethyl); 4.05 (dd, 12H, J = 4.04 Hz, crown ether protons); 1.31 (t, 3H, J = 7.06 Hz, methyl protons of the ethyl).
Step 5
-
-
Preparation: 1105 g (3.175 mol)of BPI-04, 4810 g (106.9 mol) of formamide, and 540 g (8.55 mol) of ammonium formate were added to a 10 L 3-neck bottle. The reaction mixture was heated to 165 °C under reflux for 4 hours. After cooling to room temperature, 3 L of water was added, and then the mixture was stirred for 10 minutes. After filtration, washing, and drying, 742 g of BPI-05 as a white crystalline powder was obtained in a yield of 80%. mp: 248-251 °C, HPLC: 99.78%. TLC (chloroform: methanol = 8:1) Rf = 0.55. NMR data: 1H-NMR (DMSO-d6): δ ppm: 12.06 (s, 1H, NH of the quinazoline); 8.0 (d, 1H, J = 3.28 Hz, proton of the quinazoline position 3); 7.62 (s, 1H, proton of the quinazoline position 6); 7.22 (s, 1H, proton of the quinazoline position 9); 4.25 (dd, 12H, J = 4.08 Hz, crown ether protons).
Step 6
-
-
Preparation: 337 g (1.13 mol) of BPI-05, 7.1 L of chloroform, 1.83 L (19.58mol) of POCI3 and 132 ml of N,N-dimethylformamide were added to a 10 L 3-neck bottle. The reaction mixture was stirred at reflux temperature. After dissolution, reaction completion was checked by TLC (developing solvent: chloroform: methanol = 15:1, Rf = 0.56); the reaction took approximately 8 hours to complete. Then, the reaction solution was cooled and evaporated under vacuum to dryness. The residue was dissolved in 4 L of chloroform; 4 kg of crushed ice was poured into the solution and the mixture was stirred for 0.5 hours. After separation, the aqueous phase was extracted twice with 2 L of chloroform. The organic phases were combined, 4 L of ice water was added and the pH was adjusted with 6 N NaOH to pH 8-9 while the temperature was maintained below 30 °C. After separation, the organic phase was washed with saturated NaCl, dried over anhydrous sodium sulfate and the solvents removed by vacuum evaporation. The residual solids were washed with acetone and filtered; 268 g of BPI-06 as a white crystalline powder was obtained in a yield of 77% with mp: 164-167°C and HPLC purity of 99%. NMR data: 1H-NMR (CDCl3): δ ppm: 8.89 (s, 1H, proton of the quinazoline position 2); 7.68 (s, 1H, proton of the quinazoline position 9); 7.42 (s, 1H, proton of the quinazoline position 6); 4.38-3.81 (dd, 12H, J = 3.88 Hz, crown ether protons).
Step 7
-
-
Preparation of the compound of the present invention: To a suspension of 20.8 g of BPI-06 in 500 mL of ethanol was added 25 mL of N,N-dimethylformamide and a solution of 8.98 g m-acetylene aniline in 200 mL of isopropanol. The reaction mixture was stirred at room temperature for 5 minutes until dissolved completely, and then the reaction solution was heated at reflux for 3 hours. After concentration and drying, the residual solids were dissolved in ethyl acetate, washed with water, and dried over anhydrous sodium sulfate. Thus, 27.1 g of the compound of Formula I was obtained as a white crystalline powder. NMR data: 1H-NMR (Bruker APX-400, solvent: DMSO-d6, TMS as internal standard): δ ppm: 3.58 (dd, 2H, two protons of the crown position 12); 3.60 (dd, 2H, two protons of the crown position 13); 3.73 (dd, 2H, two protons of the crown position 10); 3.80 (dd, 2H, two protons of the crown position 15); 4.30 (s, 1H, proton of the alkynyl); 4.34 (dd, 2H, two protons of the crown position 16); 4.40 (dd, 2H, two protons of the crown position 9); 7.39 (d, 1H, benzene proton at position 25); 7.46 (dd, 1H, benzene proton at position 26); 7.49 (s, 1H, proton of the quinazoline position 6); 7.82 (d, 1H, benzene proton at position 27); 7.94 (t due dd, 1H, proton of the quinazoline position 19); 8.85 (s, 1H, benzene proton at the position 23); 8.87 (s, 1H, proton of the quinazoline position 2); 11.70 (s, 1H, proton of the aromatic amine as salt); 14-16 (bs, 1H, hydrochloride), see Figure 5. NMR data: 13C-NMR (DMSO-d6), see Figure 6. Mass spectrometry (MS): Instrument: ZAB-HS, testing conditions: EI, 200°C, 700ev, MS measured molecular weight: m/z 427.
…………………………………..
https://www.google.co.in/patents/WO2013064128A1?cl=en&dq=icotinib&hl=en&sa=X&ei=1oi2UsP9LYa4rgfUzoF4&ved=0CDcQ6AEwAA
![Figure imgf000003_0002]()
Synthesis of compound 1 A
1 Synthesis of Compound 2
2
79.5g 3,4 – dihydroxybenzene nitrile, 272g of potassium carbonate, acetonitrile (6L) was added to a 10L three-necked reaction flask, and dissolved with stirring, heated to reflux and reflux was added dropwise an acetonitrile solution of the compound 1 (compound 1, 200 g; acetonitrile , 2L), and completion of the dropping, the HPLC monitoring of the completion of the reaction, the mixture was cooled to room temperature, filtered, and the solvent was removed, and the resulting solid was washed with ethyl acetate was dissolved, filtered, and the filtrate was concentrated, the resulting residue was dissolved in petroleum ether by rotary evaporation, the resulting solid was purified to give 18.9g of the compound 2.
1 LAI MR (CDC1 3-Sppm): 7.30 ~ 7.33 (m, 1H); 7.25 (s, 1H); 6.97-6.99 (d, 1H); 4.19 – 4.23 (m, 4H); 3.83 ~ 3.91 (m, 4H); 3.77 (s, 4H). MS: (M + H) +250 2 Synthesis of compound A
2 A
41.6g of compound 2 was dissolved in 580ml of acetic acid, dropwise addition of 83ml of fuming nitric acid at 30 ° C under completion of the dropping, the dropwise addition of 42ml of concentrated sulfuric acid at 30 ° C under the reaction at room temperature overnight, TLC monitoring completion of the reaction, the reaction solution was poured into ice water 4L , the precipitated solid was filtered, washed with cold water (500 mL X 2), vacuum 35 ° C and dried crude A compound 46g, isopropanol recrystallization was purified to give 33g of compound A.
1 LAI MR (CDC1 3-Sppm): 7.90 (s, 1H); 7.36 (s, 1H); 4.33 ~ 4.36 (m, 4H); 3.87 ~ 3.89 (m, 4H); 3.737 (s, 4H). Embodiment of Example 2 Synthesis of Compound B
AB
32g of compound A, 30.5g of iron powder, 5% acetic acid solution in methanol 1070ml 2L reaction flask was heated to reflux
TLC monitoring of the end of the reaction cooled and concentrated, dissolved in ethyl acetate, filtered, dried over anhydrous NaS0 4 23g of compound B. The solvent was removed.
1HNMR (d 6-DMSO-Sppm): 7.07 (s, 1H); 6.36 (s, 1H); 5.73 (s, 2H); 3.95 ~ 4.22 (m, 4H); 3.77-3.78 (m, 2H); 3.34 3.62 (m, 6H).Embodiment of Example 3 Synthesis of Compound CI
B CI
500mL three-necked flask, the Add 5g compound B, 5g v, v-dimethyl formamide dimethyl acetal and 160ml of dioxane was heated to reflux the TLC monitoring progress of the reaction, the reaction time is about 12 hours, after the end of the reaction The reaction solution was cooled to room temperature, spin-dry to give 5.8g of compound Cl.
1 LAI MR (CDCl 3-Sppm): 7.56 (s, 1H); 7.15 (s, 1H); 6.51 (s, 1H); 4.12-4.18 (m, 4H); 3.89-3.91 (m, 2H); 3.78 -3.80 (m, 6H); 3.07 (s, 6H); Example 4 Icotinib Synthesis
5 g of the compound Cl, 2.2 g inter-aminophenyl acetylene, 230ml of acetic acid was added to a 500 ml reaction flask was heated to 100 ° c,
TLC monitoring of the reaction. The end of the reaction, the reaction system spin dry methanol was added, and shock dispersion, filtration, wash with methanol, 5g Icotinib.
^ M (d 6-DMSO-5ppm): 11.98 (s, IH); 9.50 (s, IH); 8.53 (s 1H); 8.14 (s, IH); 8.04-8.05 (m, IH); 7.90-7.92 (m, IH); 7.38-7.42 (m, IH); 7.31 (s IH); 7.20-7.22 (m, IH); 4.29-4.30 (m, 4H); 4.21 (s, IH); 3.74-3.81 ( m, 4H); 3.64 (s, 4H); 1.91 (s, 3H); Synthesis Example 5 Exe hydrochloride erlotinib
Exeter for Nick for; s
700mg Icotinib Add to a 100 ml reaction flask, add 40 ml of methanol, stirred pass into the hydrogen chloride gas or concentrated hydrochloric acid, and filtered to give crude hydrochloric acid Icotinib after, and purified by recrystallization from isopropanol to give 760mg hydrochloride Icotinib.
1HNMR (d 6-DMSO-Sppm): 11.37 (s, IH); 8.87 (s, IH); 8.63 (s, IH); 7.90 (s, IH); 7.78-7.80 (d, IH); 7.48-7.52 (m, IH); 7.40-7.41 (m, 2H); 4.36-4.38 (d, 4H); 4.30 (s, IH); 3.75-3.81 (d, 4H); 3.61 (s, 4H); Example 6 Synthesis of Compound B
AB
25g of compound A, 25 g of iron powder, 3% acetic acid in methanol solution 900ml with Example 2 are the same, to give 16.6g of compound B.
Embodiment of Example 7 Synthesis of Compound B
AB
40 g of compound A, 40 g of iron powder and 7% acetic acid in methanol solution was 1200ml, in Example 2, to give 28.4g of compound B.
Example 8 Compound B Synthesis
AB
25 g of compound A, 5 g of Pd / C in 3% acetic acid in methanol solution 900ml Add 2L reaction flask, of the hydrogen, TLC monitoring of the end of the reaction, filtered, and the solvent was removed to give 17g of compound B.
Example 9 Compound B Synthesis
AB
40g of compound A, 17 g of magnesium and 5% acetic acid in methanol solution 1200ml, in Example 2, to give 25.2g of compound B. Example 10 Compound B Synthesis
AB
25 g of compound A, 32.5g of zinc powder and 5% acetic acid in methanol solution 900ml with Example 2 are the same, to give 17.1g of compound B.
Example Synthesis of compound 11 B
AB
25g of compound A, 28 g of iron powder, 5% trifluoroacetic acid in methanol solution 700ml, in Example 2, 16g of compound B.
Embodiment Example 12 Synthesis of Compound C1
3g compound B, 3G v, v-dimethyl formamide dimethyl acetal and 140ml of dioxane, reflux the reaction time is 10-11 hours, the other in the same manner as in Example 3 to give 3.2g of the compound Cl.
Example 13 Synthesis of Compound C1
8g compound B, 8G N, v-dimethyl formamide dimethyl acetal and 180ml of dioxane under reflux for a reaction time of approximately 12-13 hours, with the same manner as in Example 3 to give 8.7g of compound C. Embodiment Example 14 Synthesis of Compound CI
3g compound B, 3 g of N, N-dimethyl formamide dimethyl acetal and 140ml of toluene, the reaction time is 13-15 hours under reflux, with the same manner as in Example 3 to give 2.9g of the compound Cl.
Example 15 Synthesis of Compound C1
The same as in Example 14, except that reaction time is 10 hours, to obtain 2.6g compound Cl t
Embodiment Example 16 Synthesis of Compound C1
500mL three-necked flask, add 3 g of compound B, 3.7 g v, v-dimethylformamide, diethyl acetal and 140ml of dioxane was heated to reflux, TLC monitoring the progress of the reaction, the reaction time of approximately 11-12 hours, After completion of the reaction, the mixture was cooled to room temperature, spin-dry the reaction solution to give 2.5g of the compound Cl.
Example 17 Synthesis of Compound C1
G of compound B, 5.1 g of the N, N-dimethyl formamide di-t-butyl acetal was dissolved in 140ml dioxane was heated to reflux the TLC monitoring progress of the reaction, the reaction time of approximately 11-12 hours after the completion of the reaction, was cooled to room temperature, the reaction solution was spin-dry to give 2.6g of the compound Cl.
Embodiment Example 18 Synthesis of Compound CI
3g compound B, 4.4g N, N-dimethyl formamide diisopropyl acetal was dissolved in 140ml dioxane was heated to reflux, tlc monitoring the progress of the reaction, the reaction time of approximately 11-12 hours after the completion of the reaction, was cooled to room temperature, the reaction solution was spin-dry to give 2.4g of the compound Cl.
The implementation of the synthesis of Example 19 Icotinib
3g compound Cl, 1.3 g inter-aminophenyl acetylene, 130 ml of acetic acid was added 250 ml reaction flask and heated to 70-80
V, TLC monitoring of the reaction. Spin dry the reaction system, methanol was added, and shock dispersion, filtered, and the methanol wash was 2.8g Icotinib. Implementation of Example 20 Icotinib synthesis
C1 Icotinib
. Example 25 Icotinib Hydrochloride synthesis
Icotinib Hydrochloride
The 500mg Icotinib Add to a 100 ml reaction flask, add 30ml of ethanol was stirred under hydrogen chloride gas was passed into the after, filtered crude hydrochloride Icotinib recrystallized from isopropanol to give 515mg hydrochlorideIcotinib. Example 26 Icotinib Hydrochloride Synthesis
500mg Icotinib Add 100 ml reaction flask, add 40 ml of tetrahydrofuran was stirred under hydrogen chloride gas was passed into the after, filtered crude hydrochloride Icotinib recrystallized from isopropanol to give 500mg hydrochlorideIcotinib. EXAMPLE 27 Icotinib Hydrochloride Synthesis
500mg Icotinib Add 100 ml reaction flask, add 50 ml of isopropanol and stirred under hydrogen chloride gas was passed into the after, filtered crude hydrochloride Icotinib recrystallized from isopropanol to give 500mg hydrochloride Icotinib.
………………………………………………………………….
http://www.google.com/patents/EP2392576A1 NMR data: 1H-NMR (Bruker APX-400, solvent: DMSO-d6, TMS as internal standard): δ ppm: 3.58 (dd, 2H, two protons of the crown position 12); 3.60 (dd, 2H, two protons of the crown position 13); 3.73 (dd, 2H, two protons of the crown position 10); 3.80 (dd, 2H, two protons of the crown position 15); 4.30 (s, 1H, proton of the alkynyl); 4.34 (dd, 2H, two protons of the crown position 16); 4.40 (dd, 2H, two protons of the crown position 9); 7.39 (d, 1H, benzene proton at position 25); 7.46 (dd, 1H, benzene proton at position 26); 7.49 (s, 1H, proton of the quinazoline position 6); 7.82 (d, 1H, benzene proton at position 27); 7.94 (t due dd, 1H, proton of the quinazoline position 19); 8.85 (s, 1H, benzene proton at the position 23); 8.87 (s, 1H, proton of the quinazoline position 2); 11.70 (s, 1H, proton of the aromatic amine as salt); 14-16 (bs, 1H, hydrochloride), see Figure 5. NMR data: 13C-NMR (DMSO-d6), see Figure 6. Mass spectrometry (MS): Instrument: ZAB-HS, testing conditions: EI, 200°C, 700ev, MS measured molecular weight: m/z 427.
………………………..
NEW PATENT
WO-2013064128
Zhejiang Beta Pharma Incorporation, 浙江贝达药业有限公司
http://www.google.co.in/patents/WO2013064128A1?cl=en
General synthetic route
Compound A, the present invention is provided for availability, but are not limited to, the following synthetic route to achieve:
The present invention is to provide beta available but are not limited to, the following synthetic route is now:
A BETA
The present invention is to provide a compound C, can be used, but are not limited to, the following synthetic route to achieve:
Wherein
And are independently selected from the group consisting of methyl, ethyl, propyl or isopropyl, or
, And they are connected in common to the N atom form a 3-7 membered ring. R 3 and R4 are independently selected from the group consisting of methyl, ethyl, propyl, isopropyl, n-butyl, sec-butyl, tert-butyl, iso-butyl or benzyl group, or,
R 3 and R4 to form a 3-7 membered ring.
The present C can be used for the direct preparation of Icotinib:
Wherein
And are independently selected from the group consisting of methyl, ethyl, propyl or isopropyl, or
, And they are connected in common to the N atom form a 3-7 membered ring.
Icotinib
Icotinib Hydrochloride
Example Synthesis of compound 1 A
1 Synthesis of Compound 2
2
79.5g 3,4 – dihydroxybenzene nitrile, 272g of potassium carbonate, acetonitrile (6L) was added to a 10L three-necked reaction flask, and dissolved with stirring, heated to reflux and reflux was added dropwise an acetonitrile solution of the compound 1 (compound 1, 200 g; acetonitrile , 2L), and completion of the dropping, the HPLC monitoring of the completion of the reaction, the mixture was cooled to room temperature, filtered, and the solvent was removed, and the resulting solid was washed with ethyl acetate was dissolved, filtered, and the filtrate was concentrated, the resulting residue was dissolved in petroleum ether by rotary evaporation, the resulting solid was purified to give 18.9g of the compound 2.
1 LAI MR (CDC1 3-Sppm): 7.30 ~ 7.33 (m, 1H); 7.25 (s, 1H); 6.97-6.99 (d, 1H); 4.19 – 4.23 (m, 4H); 3.83 ~ 3.91 (m, 4H); 3.77 (s, 4H). MS: (M + H) +250 2 Synthesis of compound A
2 A
41.6g of compound 2 was dissolved in 580ml of acetic acid, dropwise addition of 83ml of fuming nitric acid at 30 ° C under completion of the dropping, the dropwise addition of 42ml of concentrated sulfuric acid at 30 ° C under the reaction at room temperature overnight, TLC monitoring completion of the reaction, the reaction solution was poured into ice water 4L , the precipitated solid was filtered, washed with cold water (500 mL X 2), vacuum 35 ° C and dried crude A compound 46g, isopropanol recrystallization was purified to give 33g of compound A.
1 LAI MR (CDC1 3-Sppm): 7.90 (s, 1H); 7.36 (s, 1H); 4.33 ~ 4.36 (m, 4H); 3.87 ~ 3.89 (m, 4H); 3.737 (s, 4H). Embodiment of Example 2 Synthesis of Compound B
AB
32g of compound A, 30.5g of iron powder, 5% acetic acid solution in methanol 1070ml 2L reaction flask was heated to reflux
TLC monitoring of the end of the reaction cooled and concentrated, dissolved in ethyl acetate, filtered, dried over anhydrous NaS0 4 23g of compound B. The solvent was removed.
1HNMR (d 6-DMSO-Sppm): 7.07 (s, 1H); 6.36 (s, 1H); 5.73 (s, 2H); 3.95 ~ 4.22 (m, 4H); 3.77-3.78 (m, 2H); 3.34 3.62 (m, 6H). Embodiment of Example 3 Synthesis of Compound CI
B CI
500mL three-necked flask, the Add 5g compound B, 5g v, v-dimethyl formamide dimethyl acetal and 160ml of dioxane was heated to reflux the TLC monitoring progress of the reaction, the reaction time is about 12 hours, after the end of the reaction The reaction solution was cooled to room temperature, spin-dry to give 5.8g of compound Cl.
1 LAI MR (CDCl 3-Sppm): 7.56 (s, 1H); 7.15 (s, 1H); 6.51 (s, 1H); 4.12-4.18 (m, 4H); 3.89-3.91 (m, 2H); 3.78 -3.80 (m, 6H); 3.07 (s, 6H); Example 4 Icotinib Synthesis
5 g of the compound Cl, 2.2 g inter-aminophenyl acetylene, 230ml of acetic acid was added to a 500 ml reaction flask was heated to 100 ° c,
TLC monitoring of the reaction. The end of the reaction, the reaction system spin dry methanol was added, and shock dispersion, filtration, wash with methanol, 5g Icotinib.
^ M (d 6-DMSO-5ppm): 11.98 (s, IH); 9.50 (s, IH); 8.53 (s 1H); 8.14 (s, IH); 8.04-8.05 (m, IH); 7.90-7.92 (m, IH); 7.38-7.42 (m, IH); 7.31 (s IH); 7.20-7.22 (m, IH); 4.29-4.30 (m, 4H); 4.21 (s, IH); 3.74-3.81 ( m, 4H); 3.64 (s, 4H); 1.91 (s, 3H);
Synthesis Example 5 Exe hydrochloride erlotinib
Exeter for Nick for; s
700mg Icotinib Add to a 100 ml reaction flask, add 40 ml of methanol, stirred pass into the hydrogen chloride gas or concentrated hydrochloric acid, and filtered to give crude hydrochloric acid Icotinib after, and purified by recrystallization from isopropanol to give 760mg hydrochloride Icotinib.
1HNMR (d 6-DMSO-Sppm): 11.37 (s, IH); 8.87 (s, IH); 8.63 (s, IH); 7.90 (s, IH); 7.78-7.80 (d, IH); 7.48-7.52 (m, IH); 7.40-7.41 (m, 2H); 4.36-4.38 (d, 4H); 4.30 (s, IH); 3.75-3.81 (d, 4H); 3.61 (s, 4H);
Example 18 Synthesis of Compound CI
3g compound B, 4.4g N, N-dimethyl formamide diisopropyl acetal was dissolved in 140ml dioxane was heated to reflux, tlc monitoring the progress of the reaction, the reaction time of approximately 11-12 hours after the completion of the reaction, was cooled to room temperature, the reaction solution was spin-dry to give 2.4g of the compound Cl.
The implementation of the synthesis of Example 19 Icotinib
3g compound Cl, 1.3 g inter-aminophenyl acetylene, 130 ml of acetic acid was added 250 ml reaction flask and heated to 70-80
V, TLC monitoring of the reaction. Spin dry the reaction system, methanol was added, and shock dispersion, filtered, and the methanol wash was 2.8g Icotinib. Implementation of Example 20 Icotinib synthesis
C1 Icotinib
8g compound Cl, 3.5g inter-aminophenyl acetylene, dissolved in 380ml of acetic acid, heated to 100-120 ° C, TLC monitoring of the reaction. Spin dry the reaction system, by adding ethanol shock dispersion, filter, the ethanol wash 7.2g Icotinib. Implementation of Example 21 Icotinib Synthesis
The C1 Exeter erlotinib reaction temperature of 120-15CTC Example 4 was 2.2 g Icotinib.
Example 22 Icotinib Synthesis
3g compound Cl, 1.8 g inter-aminophenyl acetylene and 130 ml of acetic acid was added 250 ml reaction flask and heated to 90-100C, TLC monitoring of the reaction. Spin dry the reaction system, isopropanol shock dispersion, filtration, isopropyl alcohol wash was 2.9g Icotinib.
The implementation of the synthesis of Example 23 Icotinib
3G compound CI and 1.3 g of m-aminophenyl acetylene dissolved in 130ml of formic acid was heated to 80-90 ° C, TLC monitoring of the reaction. Spin dry the reaction system, methanol was added, and shock dispersion, filtered, and the methanol wash was 2.7g Icotinib.
Example 24 Icotinib synthesis
3g of compound C1 and 1.3g aminophenyl acetylene dissolved in 130ml of trifluoroacetic acid was heated to 70-80 ° C, TLC monitoring of the reaction. Spin dry the reaction system, methanol was added, and shock dispersion, filtered, and the methanol wash was 2.7g Icotinib. Example 25 Icotinib Hydrochloride synthesis
Icotinib Hydrochloride
The 500mg Icotinib Add to a 100 ml reaction flask, add 30ml of ethanol was stirred under hydrogen chloride gas was passed into the after, filtered crude hydrochloride Icotinib recrystallized from isopropanol to give 515mg hydrochloride Icotinib. Example 26 Icotinib Hydrochloride Synthesis
500mg Icotinib Add 100 ml reaction flask, add 40 ml of tetrahydrofuran was stirred under hydrogen chloride gas was passed into the after, filtered crude hydrochloride Icotinib recrystallized from isopropanol to give 500mg hydrochloride Icotinib. EXAMPLE 27 Icotinib Hydrochloride Synthesis
Exeter erlotinib erlotinib hydrochloride Exeter
500mg Icotinib Add 100 ml reaction flask, add 50 ml of isopropanol and stirred under hydrogen chloride gas was passed into the after, filtered crude hydrochloride Icotinib recrystallized from isopropanol to give 500mg hydrochloride Icotinib. Example 28 Icotinib Hydrochloride synthesis
Icotinib
Icotinib Hydrochloride
References
- Sordella, R. (20 August 2004). “Gefitinib-Sensitizing EGFR Mutations in Lung Cancer Activate Anti-Apoptotic Pathways”. Science 305(5687): 1163–1167. doi:10.1126/science.1101637. PMID 15284455.
- Shi, Yuankai; Zhang, Li; Liu, Xiaoqing; Zhou, Caicun; Zhang, Li; Zhang, Shucai; Wang, Dong; Li, Qiang; Qin, Shukui; Hu, Chunhong; Zhang, Yiping; Chen, Jianhua; Cheng, Ying; Feng, Jifeng; Zhang, Helong; Song, Yong; Wu, Yi-Long; Xu, Nong; Zhou, Jianying; Luo, Rongcheng; Bai, Chunxue; Jin, Yening; Liu, Wenchao; Wei, Zhaohui; Tan, Fenlai; Wang, Yinxiang; Ding, Lieming; Dai, Hong; Jiao, Shunchang; Wang, Jie; Liang, Li; Zhang, Weimin; Sun, Yan. “Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trial”. The Lancet Oncology 14 (10): 953–961. doi:10.1016/s1470-2045(13)70355-3.
- Tan, Fenlai; Gu, Aiqin; Zhang, Yiping; Jiao, Shun Chang; Wang, Chang-li; He, Jintao; Jia, Xueke; Zhang, Li; Peng, Jiewen; Wu, Meina; Ying, Kejing; Wang, Junye; Ma, Kewei; Zhang, Shucai; You, Changxuan; Ding, Lieming; Wang, Yinxiang; Shen, Haijiao; Wan, Jiang; Sun, Yan (2013). “Safety and efficacy results of a phase IV, open-label, multicenter, safety-monitoring study of icotinib in treating advanced non-small cell lung cancer (NSCLC): ISAFE study”. ASCO 2013 Meeting: e19161.
- Chen, Xiaofeng; Zhu, Quan; Liu, Yiqian; Liu, Ping; Yin, Yongmei; Guo, Renhua; Lu, Kaihua; Gu, Yanhong; Liu, Lianke; Wang, Jinghua; Wang, Zhaoxia; Røe, Oluf Dimitri; Shu, Yongqian; Zhu, Lingjun; Chellappan, Srikumar P. (16 May 2014). “Icotinib Is an Active Treatment of Non-Small-Cell Lung Cancer: A Retrospective Study”. PLoS ONE 9 (5): e95897.doi:10.1371/journal.pone.0095897.
| WO2007138613A2 * |
12 Mar 2007 |
6 Dec 2007 |
Venkateshappa Chandregowda |
A process for synthesis of [6,7-bis-(2-methoxyethoxy)-quinazolin-4-yl]-(3-ethynylphenyl)amine hydrochloride |
| WO2010003313A1 |
7 Jul 2009 |
14 Jan 2010 |
Zhejiang Beta Pharma Inc. |
Icotinib hydrochloride, synthesis, crystallographic form, medical combination, and uses thereof |
| CN1305468C |
29 May 2003 |
21 Mar 2007 |
中国人民解放军第三○二医院 |
Bolengsu compound and its preparation, medicine composition and use |
| US7078409 |
26 Mar 2003 |
18 Jul 2006 |
Beta Pharma, Inc. |
Fused quinazoline derivatives useful as tyrosine kinase inhibitors |
| Patent |
Submitted |
Granted |
| Icotinib Hydrochloride, Synthesis, Crystalline Forms, Pharmaceutical Compositions, and Uses Thereof [US2011182882] |
2011-07-28 |
|
| Fused quinazoline derivatives useful as tyrosine kinase inhibitors [US7078409] |
2004-03-11 |
2006-07-18 |
Filed under:
cancer,
PHASE1 Tagged:
EGFR,
EGFR. Qingwen Zhang,
icotinib,
PHASE1,
TLC ![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
 Blinatumomab linking a T cell to a malignant B cell.
Blinatumomab linking a T cell to a malignant B cell.












































![4-fluoro-5-[[(2S)-2-methyl-1,4-diazepan-1-yl]sulfonyl]isoquinoline NMR spectra analysis, Chemical CAS NO. 223645-67-8 NMR spectral analysis, 4-fluoro-5-[[(2S)-2-methyl-1,4-diazepan-1-yl]sulfonyl]isoquinoline H-NMR spectrum](http://pic11.molbase.net/nmr/nmr_image/2014-11-28/002/466/2466109_1h.png)
![4-fluoro-5-[[(2S)-2-methyl-1,4-diazepan-1-yl]sulfonyl]isoquinoline NMR spectra analysis, Chemical CAS NO. 223645-67-8 NMR spectral analysis, 4-fluoro-5-[[(2S)-2-methyl-1,4-diazepan-1-yl]sulfonyl]isoquinoline C-NMR spectrum](http://pic11.molbase.net/nmr/nmr_image/2014-11-28/002/466/2466109_13c.png)



















































































 SIGNIFOR is supplied as a sterile solution in a single-dose, 1 mL colorless glass ampule containing pasireotide in 0.3 mg/mL, 0.6 mg/mL, or 0.9 mg/mL strengths for subcutaneous injection.
SIGNIFOR is supplied as a sterile solution in a single-dose, 1 mL colorless glass ampule containing pasireotide in 0.3 mg/mL, 0.6 mg/mL, or 0.9 mg/mL strengths for subcutaneous injection.









 now taken over by sun
now taken over by sun