InterMune has received breakthrough therapy designation from the US Food and Drug Administration (FDA) for its pirfenidone, an investigational treatment for adult patients with idiopathic pulmonary fibrosis (IPF).
The company had submitted a new drug application to the FDA in May for pirfenidone and noted a target FDA review of six months under the Prescription Drug User Fee Act.
http://www.pharmaceutical-technology.com/news/newsfda-grants-breakthough-therapy-designation-for-intermunes-pirfenidone-4321293
InterMune’s Esbriet (Pirfenidone), an orally active, anti-fibrotic agent that inhibits the synthesis of TGF-beta, is currently seeking approval from the U.S. Food and Drug Administration (FDA) for the treatment of adult patients with idiopathic pulmonary fibrosis (IPF), a progressive and eventually fatal lung disease. On May 27, 2014 Brisbane, California-based InterMune resubmitted its pirfenidone New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) in response to a Complete Response Letter (CRL) received in May 2010.
On July 17, 2014, Pirfenidone was awardedbreakthrough therapy designation by the FDA. If the FDA approves it within six months, Pirfenidonecould be sold in the United States in the first quarter of 2015.
InterMune licensed pirfenidone from Marnac, Inc. and its co-licensor, KDL GmbH, in 2002 and in 2007 purchased from Marnac and KDL the rights to sell the compound in the United States, Europe and other territories except in Japan, Taiwan and South Korea where rights to the molecule were licensed by Marnac and KDL to Shionogi & Co. Ltd. of Japan.
Pirfenidone is the only commercially approved drug for the treatment of mild to moderate idiopathic pulmonary fibrosis(IPF) in the world and is now approved in the EU, Norway, Iceland, Canada, Japan, China, India, South Korea, Argentina and Mexico.
![]()
In Japan it is marketed as Pirespa (ピレスパ) by Shionogi & Co since 2008.
![]()
In 2011 it was approved for use in Europe for IPF under the trade nameEsbriet, where the drug is priced in the range of $33,000 to $47,000 per year, depending on the country.
![]()
In October 2010, the Indian Company Cipla launched it as Pirfenex.
![]()
In September 2011, the China Food and Drug Administration (CFDA) granted Shanghai genomics (上海睿星基因技术有限公司), the wholly owned subsidiary of Japan-based GNI Group Ltd, with approval of pirfenidone (F647) under the trade name Etuary (艾思瑞) in China. Etuary (pirfenidone, F647) was manufactured by GNI’s affiliate Beijing Continent Pharmaceuticals (北京康蒂尼药业有限公司).
The U.S. Food and Drug Administration (FDA) declined to approve pirfenidone in 2010 because InterMune’s two previous Phase III studies ( known as CAPACITY) of Esbriet brought mixed results, insisting on another Phase III trial after an advisory committee recommended approval of the drug, but by a 9–3 margin.
In February 2014, InterMune said its latest Esbriet the phase III “Ascend” study of 555 IPF patients showed strong and positive results. Pirfenidone improved lung function and slowed the progression of IPF — meeting its primary endpoint of reducing the risk of a meaningful decline in forced vital capacity compared to the placebo group from baseline at week 52.
Pirfenidone is still under investigation for the treatment of IPF in the United States and has not been approved by the FDA.
Esbriet (Pirfenidone) is the only product marketed by InterMune. Revenue from the drug was about $70.3 million in 2013. The company recorded Esbriet sales of $30.3 million in the first quarter of 2014. Esbriet sales in 2014 are expected in the range of $130−$140 million.
![]()
Pirfenidone (INN, BAN) is a drug developed by several companies worldwide, including InterMune Inc., Shionogi Ltd., and GNI Group Ltd., for the treatment of idiopathic pulmonary fibrosis (IPF). In 2008, it was first approved in Japan for the treatment of IPF after clinical trials, under the trade name of Pirespa by Shionogi & Co. In October 2010, the Indian Company Cipla launched it as Pirfenex. In 2011, it was approved for use in Europe for IPF under the trade name Esbriet.[2] The proposed trade name in the US is also Esbriet. In September 2011, the Chinese State Food and Drug Administration provided GNI Group Ltd with new drug approval of pirfenidone in China,[3] and later manufacture approval in 2013 under the trade name of Etuary.[4]
In 2014 it was approved in México under the name KitosCell LP, indicated for pulmonary fibrosis and liver fibrosis.[5] There is also a topical form created for the treatment of abnormal wound healing processes.[6]
![]()
![]()
Mechanism of action
Pirfenidone has well-established antifibrotic and anti-inflammatory properties in various in vitro systems and animal models offibrosis.[7] A number of cell-based studies have shown that pirfenidone reduces fibroblast proliferation,[8][9][10][11] inhibits TGF-βstimulated collagen production[8][9][12][13][14] and reduces the production of fibrogenic mediators such as TGF-β.[10][13] Pirfenidone has also been shown to reduce production of inflammatory mediators such as TNF-α and IL-1β in both cultured cells and isolatedhuman peripheral blood mononuclear cells.[15][16] These activities are consistent with the broader antifibrotic and anti-inflammatoryactivities observed in animal models of fibrosis.
Preclinical studies
Studies in models of fibrosis
In animal models, pirfenidone displays a systemic antifibrotic activity and has been shown to reduce biochemical and histopathological indices of fibrosis of the lung, liver, heart and kidney.[7]
Pirfenidone demonstrates a consistent antifibrotic effect in several animal models of pulmonary fibrosis.[17][18][19][20][21] Of these, the bleomycin model is the most widely used model of pulmonary fibrosis. In this model, bleomycin administration results in oxidative stress and acute inflammation, with the subsequent onset of pulmonary fibrosis in a number of animal species including the mouse and hamster.[7][19] Numerous studies have demonstrated that pirfenidone attenuates bleomycin-induced pulmonary fibrosis.[17][18][21][22][23][24] One study investigated the effect of pirfenidone over a 42-day period after repeated bleomycin administration.[18] Administration of pirfenidone minimised early lung oedema and pulmonary fibrosis when treatment was initiated concurrently with lung damage. This study evaluated pulmonary protein expression and found pirfenidone treatment normalised expression of pro-inflammatory and fibrogenic proteins. Similar reductions in pulmonary fibrosis were observed when pirfenidone treatment was delayed until pulmonary fibrosis was established and progressing,[17] i.e. when administered in a therapeutic as opposed to a prophylactic treatment regimen.
The antifibrotic effect of pirfenidone has been further established in animal models of cardiac,[25][26][27] renal,[28][29] and hepatic[8][30][31] fibrosis. In these models, pirfenidone demonstrated a consistent ability to reduce fibrosis and the expression of fibrogenic mediators.
Pharmacokinetics
Pirfenidone is administered orally. Though the presence of food significantly reduces the extent of absorption, the drug is to be taken after food, to reduce the nausea and dizziness associated with the drug. The drug is around 60% bound to plasma proteins, especially to albumin.[32] Up to 50% of the drug is metabolized by hepatic CYP1A2 enzyme system to yield 5-carboxypirfenidone, the inactive metabolite. Almost 80% of the administered dose is excreted in the urine within 24 hours of intake.[32]
Clinical trials in Idiopathic Pulmonary Fibrosis (IPF)
The clinical efficacy of pirfenidone has been studied in three Phase III, randomized, double-blind, placebo-controlled studies in patients with IPF.[33][34]
The first Phase III clinical trial to evaluate the efficacy and safety of pirfenidone for the treatment of patients with IPF was conducted in Japan. This was a multicentre, randomised, double-blind, trial, in which 275 patients with IPF were randomly assigned to receive pirfenidone 1800 mg/day (110 patients), pirfenidone 1200 mg/day (56 patients), or placebo(109 patients), for 52 weeks. Pirfenidone 1800 or 1200 mg/day reduced the mean decline in vital capacity from baseline to week 52 compared with placebo. Progression-free survival was also improved with pirfenidone compared with placebo.[33]
The CAPACITY (004 & 006) studies were randomized, double-blind, placebo-controlled Phase III trials in eleven countries across Europe, North America, and Australia.[34] Patients with IPF were randomly assigned to treatment with oral pirfenidone or placebo for a minimum of 72 weeks.[34] In study 004, pirfenidone reduced decline in forced vital capacity(FVC) (p=0.001). Mean change in FVC at week 72 was –8.0% (SD 16.5) in the pirfenidone 2403 mg/day group and –12.4% (SD 18.5) in the placebo group, a difference of 4.4% (95% CI 0.7 to 9.1). Thirty-five (20%) of 174 versus 60 (35%) of 174 patients, respectively, had an FVC decline of at least 10%. In study 006, the difference between groups in FVC change at week 72 was not significant (p=0.501). Mean change in FVC at week 72 was –9.0% (SD 19.6) in the pirfenidone group and –9.6% (19.1) in the placebo group. The difference between groups in change in predicted FVC at week 72 was not significant (0.6%, 95% CI –3.5 to 4.7).[34]
In May, 2014, the results of ASCEND studies (Phase III) were published. ASCEND is a randomized, double-blind, placebo-controlled trial that enrolled 555 patients. The results confirmed observations from previous clinical studies that pirfenidone significantly reduced IPF disease progression as measured by change in percent predicted forced vital capacity (FVC) from Baseline to Week 52 (rank ANCOVA p<0.000001). In addition, significant treatment effects were shown on both of the key secondary endpoints of six-minute walk test distance change (p=0.0360) and progression-free survival (p=0.0001). A pre-specified analysis of the pooled population (N=1,247) from the combined ASCEND and CAPACITY studies (taking CAPACITY mortality data through Week 52) showed that the risk of all-cause mortality was reduced by 48% in the pirfenidone group compared to the placebo group (HR=0.52, log rank p=0.0107)[35] .
A review by the Cochrane Collaboration concluded that pirfenidone appears to improve progression-free survival and, to a lesser effect, pulmonary function in patients with IPF.[36]Randomised studies comparing non-steroid drugs with placebo or steroids in adult patients with IPF were included. Four placebo-controlled trials of pirfenidone treatment were reviewed, involving a total of 1155 patients. The result of the meta-analysis showed that pirfenidone significantly reduces the risk of disease progression by 30%. In addition, meta-analysis of the two Japanese studies confirmed the beneficial effect of pirfenidone on the change in VC from baseline compared with placebo.[36]
Indication
In Europe, pirfenidone is indicated for the treatment of mild-to-moderate idiopathic pulmonary fibrosis. It was approved by the European Medicines Agency (EMA) in 2011.[2] In October 2008, it was approved for use in Japan, in India in 2010, and in China in 2011 (commercial launch in 2014).
In Mexico it has been approved on a gel[37] form for the treatment of scars and fibrotic tissue [38] and has proven to be effective in the treatment of skin ulcers, such as diabetic foot.
Other research done shows that Pirfenidone can be an effective anti-fibrotic treatment [39] for chronic liver fibrosis.[40]
Adverse effects
Gastrointestinal
Pirfenidone is frequently associated with gastrointestinal side effects such as dyspepsia, nausea, gastritis, gastroesophageal reflux disease (GERD) and vomiting.To reduce the severity of these reactions, pirfenidone is to be taken after meals.[32]
Skin
Pirfenidone is known to cause photosensitivity reactions, rash, pruritus and dry skin. Patients are usually advised to avoid direct exposure to sunlight, including sun lamps, and to use protective clothing and sunscreen agents. Continuing photosensitivity reactions are usually managed by dose adjustment and temporary discontinuation of treatment if required, along with local symptomatic treatment.[32]
Hepatic dysfunction
Pirfenidone can increase hepatic enzyme levels, especially those of aspartate transaminase (AST), alanine transaminase (ALT) and gamma-glutamyl transpeptidase (GGT); periodic monitoring of hepatic enzyme levels is required during therapy: once before the initiation of therapy, monthly monitoring until 6 months after initiation of therapy, and 3 monthly thereafter. Extra precaution is required while prescribing the drug in patients with hepatic impairment and in patients who are concomitantly taking a CYP1A2 inhibitor. The drug is contraindicated in patients who have severe hepatic impairment.[32]
Dizziness and fatigue
Dizziness and fatigue have been reported in patients undergoing pirfenidone treatment. Dizziness typically resolves, although patients should know how they react to pirfenidone before undertaking activities that need mental alertness or coordination. If severe, dose adjustment or treatment discontinuation may be required.[32]
Weight loss
Weight loss has been reported in patients treated with pirfenidone. Doctors should monitor patients’ weight and encourage increased calorific intake if necessary.[32]
Interactions
Most drug interactions are mediated by various cytochrome P450 (CYP) enzymes.[32]
CYP1A2 inhibitors
Since Pirfenidone is metabolised through the CYP1A2 enzyme pathway, any drug which inhibits this enzyme is likely to precipitate the toxicity of pirfenidone: concomitant therapy is to be avoided. Fluvoxamine is contraindicated in patients who are on treatment with pirfenidone. Other inhibitors of CYP1A2 such as ciprofloxacin, amiodarone and propafenoneshould be used with caution.[32]
Other CYP inhibitors
Some pirfenidone is also metabolized by CYP enzymes other than CYP1A2. Consequently, strong inhibitors of other CYP systems such as fluconazole (CYP2C9), chloramphenicol(CYP2C19), fluoxetine and paroxetine (both CYP2D6) should be used with caution.[32]
CYP1A2 inducers
Moderate inducers of CYP1A2 such as omeprazole should be used with caution since they might reduce the circulating plasma levels of the drug.[32]
Cigarette smoking
Cigarette smoking causes increased clearance of pirfenidone by inducing CYP1A2, thereby decreasing exposure to the drug. Patients must be advised to abstain from cigarette smoking while on therapy with pirfenidone.[32]
Regulatory progress
In May 2010, the U.S. Food and Drug Administration declined to approve the use of pirfenidone for the treatment of idiopathic pulmonary fibrosis, requesting additional clinical trials.[41] In December 2010 an advisory panel to the European Medicines Agency recommended approval of the drug.[2] In February 2011, the European Commission (EC) has granted marketing authorisation in all 27 EU member states and China FDA granted approval in September, 2011. Afterwards, a randomised, Phase III trial (the ASCEND study) has been completed in the U.S. in 2014.[42] Application for the U.S. regulatory approval is expected in 2014.
In Mexico it has been approved in gel for the treatment of chronic wounds and skin injuries and the oral form it is approved for the treatment of Pulmonary Fibrosis and Liver fibrosis.
Pirfenidone is a non-peptide synthetic molecule with a molecular weight of 185.23 daltons. Its chemical elements are expressed as CI2HHNO, and its structure is known. The synthesis of pirfenidone has been worked out. Pirfenidone is manufactured and being evaluated clinically as a broad- spectrum anti-fibrotic drug. Pirfenidone has anti-fibrotic properties via: decreased TNF-α expression, decreased PDGF expression, and decreased collagen expression. Several pirfenidone Investigational New Drug Applications (INDs) are currently on file with the U.S. Food and Drug Administration. Phase II human investigations have been initiated or completed for pulmonary fibrosis, renal glomerulosclerosis, and liver cirrhosis. There have been other Phase II studies that used pirfenidone to treat benign prostate hypertrophy, hypertrophic scarring (keloids), and rheumatoid arthritis.
One important use of pirfenidone is known to be providing therapeutic benefits to patients suffering from fibrosis conditions such as Hermansky-Pudlak Syndrome (HPS) associated pulmonary fibrosis and idiopathic pulmonary fibrosis (IPF). Pirfenidone demonstrates a pharmacologic ability to prevent or remove excessive scar tissue found in fibrosis associated with injured tissues including that of lungs, skin, joints, kidneys, prostate glands, and livers. Published and unpublished basic and clinical research suggests that pirfenidone may safely slow or inhibit the progressive enlargement of fibrotic lesions, remove pre-existing fibrotic lesions, and prevent formation of new fibrotic lesions following tissue injuries.
It is understood that one mechanism by which pirfenidone exerts its therapeutic effects is by modulating cytokine actions. Pirfenidone is a potent inhibitor of fibrogenic Attorney Docket: 30481/30033 A cytokines and TNF-α. It is well documented that pirfenidone inhibits excessive biosynthesis or release of various fibrogenic cytokines such as TGF-βl, bFGF, PDGF, and EGF. Zhang S et ah, Australian New Eng. J. OphthaL, 26:S74-S76 (1998). Experimental reports also show that pirfenidone blocks the synthesis and release of excessive amounts of TNF-α from macrophages and other cells. Cain et al., Int. J. Immunopharm. , 20:685-695 (1998).
Pirfenidone has been studied in clinical trials for use in treatment of IPF. Thus, there is a need for a synthetic scheme that provides pirfenidone having sufficient purity as an active pharmaceutical ingredient (API) and involves efficient and economical processes. Prior batches of pirfenidone were shown to have residual solvent traces of ethyl acetate (e.g., about 2 ppm) and butanol.
http://www.google.com/patents/EP2440543A2?cl=en
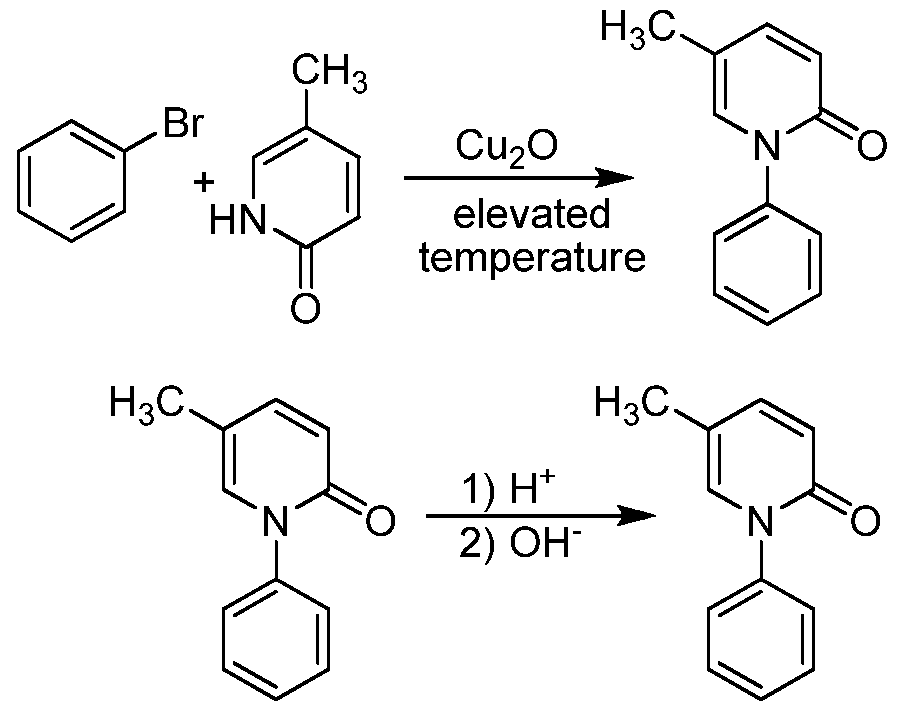
improved process for preparing pirfenidone. The process involves using a cuprous oxide catalyst to couple 5-methyl-2-pyridone and bromobenzene in an organic solvent. Without intending to be limited by any particular theory, it is believed that the purity of the bromobenzene is important, as amounts of a dibromobenzene impurity in the bromobenzene can lead to dimer-type byproducts, which can complicate the Attorney Docket: 30481/30033 A purification of the resulting pirfenidone.
These dimer-type byproducts cannot be in a product intended as to be marketed as an active pharmaceutical ingredient (API), and they are difficult to remove from the intended pirfenidone product. Thus, the bromobenzene used in the disclosed processes preferably have an amount of dibromobenzene of less than about 0.15% by weight or molar ratio, and more preferably less than about 0.1% by weight or molar ratio or less than 0.05% by weight or molar ratio.
![]()
http://www.google.com/patents/US8519140
EXAMPLES
Coupling of Bromobenzene and 5-Methyl-2-pyridone
5-Methyl-2-pyridone (1.0 equivalents), potassium carbonate (1.2 equivalents), copper(I) oxide (0.05 equivalents), bromobenzene (1.8 equivalents, with a purity of at least 98%, preferably at least 99%, or at least 99.8%), and dimethyl formamide (2.0 volume equivalents) were charged into an inert reactor. This mixture was heated to 125° C. for about 18 hours. A sample was collected and analyzed for reaction completion. If reaction completion was not satisfactory, the reaction was maintained at 125° C. for an additional 2 hours. The reaction mixture was then cooled to 25° C. to form a slurry.
The resulting slurry was filtered in a Nutsche filter in order to remove salts. The filter cake was rinsed twice with toluene. The mother liquor and process liquor were collected in Vessel (A). A sodium chloride solution (15%) was charged into the product solution. The pH was adjusted to greater than or equal to 11.5 using a 32% sodium hydroxide solution. The mixture was then agitated. After agitation was stopped, the mixture was allowed to settle for at least 30 minutes to allow the two phases to separate. The organic layer was separated and the aqueous layer was extracted with toluene. The toluene extraction was added to the organic layer. The combined organics were then washed with a 15% sodium chloride solution and agitated for at least 15 minutes. The agitation was stopped and the layers were allowed to settle for at least 30 minutes. The organic layer was separated from the aqueous layer, and then carbon treated by flowing it through Zeta Carbon filters for 2 hours at 20-25° C. The carbon treated solution was then concentrated under vacuum to remove all water and much of the toluene.
Heptanes were then added to the concentrated solution, and it was heated to about 80° C. The solution was slowly cooled to about 0° C. over at least 7 hours. The pirfenidone precipitated out of the solution, was collected by filtration and dried, using a Nutsche filter/drier. The pirfenidone cake was washed twice with a mixture of toluene and heptanes (at 0° C.), then vacuum dried at a temperature of about 42° C. The crude pirfenidone was formed in about 85% yield.
Crystallization of Pirfenidone
Pirfenidone, a 32% hydrochloride solution, and deionized water were charged in an inert reactor. The mixture was heated to about 45° C., then a 32% sodium hydroxide solution was titrated into the mixture until the pH was at least 11. The temperature of the mixture was maintained at about 45° C. during the titration. Upon reaching the pH of at least 11, the mixture was then cooled slowly to 5° C., over the course of at least 2 hours. The pirfenidone crystallized from this cooled solution and was isolated in a Nutsche filter/drier. The pirfenidone cake was washed twice with deionized water (at 5° C.). The pirfenidone was then vacuum dried in the filter/drier at a temperature of about 45° C. The pirfenidone was also milled through a loop mill in order to reduce the particle size to less than 150 μm.
The resulting pirfenidone was then analyzed and the only residual solvents observed were toluene and heptanes at about 10 to 13 ppm. No ethyl acetate or butanol was detected in the pirfenidone. The amount of bis-conjugate in the purified pirfenidone was 0.03% or less. All impurities of the purified pirfenidone were less than about 0.05%.
…………………………….
http://www.google.com/patents/CN1218942C?cl=en
fibrotic diseases such as renal fibrosis and cirrhosis, myocardial fibrosis is a class of serious harm to human life and health of important diseases, as well as people living with global industrialization, changes in diet, the incidence of fibrotic diseases is gradually increased correspondingly, many domestic and foreign scholars fibrosis links from chemical compounds, natural compounds, biologics, gene therapy and other different areas of a large number of anti-fibrotic compounds studied. So far, the pyridone compound has been found that a class of effective antifibrotic compound.
U.S. Patent US3839346, US4052509A discloses a pyridone compound of structural formula are available (O) of the general formula 1 – mono-substituted phenyl-5 – methyl -2 (1H)-pyridone.
Image not available. View PDF Wherein the number of the substituent R is 0 or 1, R represents a nitro substituent species, a chlorine atom, an alkyl group, a methoxy group; such pyridones have anti-inflammatory, antipyretic, lower serum uric acid levels, pain and so on.
In addition, U.S. Patent (US3839346) discloses a process approach is to formula (IV), 5 – methyl -2 (1H)-pyridone as raw materials, and formula (V) monosubstituted phenyl iodide, the reaction and generating (O) type 1 – benzene substituted-5 – methyl -2 (1H) pyridone compound, the reaction process is as follows: Image not available. View PDF Chinese Patent (1086514A) discloses a process for preparing formula (IV) method is based on formula (IV) 1 – nitrile-1 – butene and (VII) formula 1,1 – bis dimethyl ether as amine starting material, the reaction of (VIII) Formula 1 – dimethylamine -2 – methyl-4 – cyano-1 ,3 – butadiene intermediates in acid conditions and then cyclized to generate ( IV ‘) formula and formula (IV) of the desired compound, the reaction process is as follows:Image not available. View PDF Although these methods to some of the previous methods were further improved, but there are still formula (VI) compound is unstable, prone to aggregation, (VII) is not easy to obtain the compound of formula shortcomings.
On the other hand, ORGANIC SYNTHESES Vol.78, 51 discloses the compound (II) Preparation of Compound (IV) method Image not available. View PDF SUMMARY OF THE INVENTION For the above-mentioned disadvantages of the prior art, the present invention is one of the technical solution is to provide a class of anti-fibrosis effect, and organ and has a wide applicability antifibrotic pyridinone compound; technical solution of the present invention The second program is to provide an easy to use on the market too, and the starting material for production of stable molecules antifibrotic pyridone compound process method.
……………………………..
![A Simple Synthesis of Pirfenidone (Esbriet,Pirespa,ピレスパ,Pirfenex, Etuary), InterMune's idiopathic pulmonary fibrosis Drug 特发性肺纤维化药物吡非尼酮(艾思瑞)的简单制备方法]()
………………………
Pirfenidone
By condensation of 5-methyl-2- (1H) -pyridone (I) with iodobenzene (II) by means of K2CO3 and Copper powder at reflux temperature.
![]()
Casta Lv r, J .; Blancafort, P .; Pirfenidone. Drugs Fut 1977, 2, 6,
US 3839346; ZA 7309472
CA 1049411;. DE 2555411; US 3974281
| WO2002085858A1 * |
Apr 19, 2002 |
Oct 31, 2002 |
Asahi Glass Co Ltd |
Process for producing purified piperidine derivative |
| WO2003014087A1 * |
Aug 6, 2002 |
Feb 20, 2003 |
Asahi Glass Co Ltd |
Process for preparation of 5-methyl-1-phenyl-2(1h) -pyridinone |
| WO2008147170A1 * |
May 29, 2008 |
Dec 4, 2008 |
Armendariz Borunda Juan Socorr |
New process of synthesis for obtaining 5-methyl-1-phenyl-2 (ih) -pyridone, composition and use of the same |
| Hegde et al., “17. Pirfenidone (Idiopathic Pulmonary Fibrosis), Chapter 28 To Market, To Market-2008,” Ann Rep Med Chem, vol. 44 (2009). |
| 2 |
|
Hegde et al., “17. Pirfenidone (Idiopathic Pulmonary Fibrosis), Chapter 28 To Market, To Market—2008,” Ann Rep Med Chem, vol. 44 (2009). |
| 3 |
|
International Search Report from corresponding International Application No. PCT/US2010/037090, dated Mar. 1, 2011. |
| 4 |
|
Ma et al., “Synthesis of pirfenidone,” Zhongguo Yiyao Gongye Zazhi, 37(6):372-373 as summarized in Liu et al., “Synthetic Approaches to the 2008 New Drugs,” Mini-Reviews in Medicinal Chemistry, 9:1655-75 (2009). |
| 5 |
* |
Vogel, A., Practical Organic Chemistry, 3d ed., London, Longman Group, 1974, pp. 44-45 and 122-127. |
| 6 |
|
Wu et al., Tissue distribution and plasma binding of a novel antifibrotics drug pifenidone in rats, Asian J. Pharmadynamics and Pharmacokinetics, 6(4):351-6 (2006). |
| 7 |
|
Zhang et al., Pirfenidone reduces fibronectin synthesis by cultured human retinal pigment epithelial cells, Aust. N Z J Ophthalmol., 26 Suppl 1:S74-6 (1998). |
References
- “Esbriet 267 mg hard capsules”. electronic Medicines Compendium. Intermune UK & I Ltd. 3 January 2014. Retrieved 6 March 2014.
- “InterMune: Pirfenidone”. InterMune.
- “China SFDA Approves F647/pirfenidone for the Treatment of Idiopathic Pulmonary Fibrosis”. Press Release. EvaluatePharma. 2011-09-22.
- “GNI Group receives manufacture approval for F647 from China FDA for first idiopathic pulmonary fibrosis drug in China”. Press Release. 2014-01-06.
- kitoscelllp.com
- Jump up^ Coadjuvant treatment with surgery and pirfenidone in sever facial trauma due to dog bite. The journal of Craniofacial Surgery, volume 24, Number 2, March 2013
- ^ Jump up to:a b c Schaefer CJ, Ruhrmund DW, Pan L, Seiwert SD, Kossen K (June 2011). “Antifibrotic activities of pirfenidone in animal models”. Eur Respir Rev 20 (120): 85–97.doi:10.1183/09059180.00001111. PMID 21632796.
- ^ Jump up to:a b c Di Sario A, Bendia E, Svegliati Baroni G, Ridolfi F, Casini A, Ceni E, Saccomanno S, Marzioni M, Trozzi L, Sterpetti P, Taffetani S, Benedetti A (November 2002). “Effect of pirfenidone on rat hepatic stellate cell proliferation and collagen production”. J. Hepatol.37 (5): 584–91. doi:10.1016/S0168-8278(02)00245-3. PMID 12399223.
- ^ Jump up to:a b Hewitson TD, Kelynack KJ, Tait MG, Martic M, Jones CL, Margolin SB, Becker GJ (2001). “Pirfenidone reduces in vitro rat renal fibroblast activation and mitogenesis”. J. Nephrol. 14 (6): 453–60. PMID 11783601.
- ^ Jump up to:a b Lin X, Yu M, Wu K, Yuan H, Zhong H (August 2009). “Effects of pirfenidone on proliferation, migration, and collagen contraction of human Tenon’s fibroblasts in vitro”.Invest. Ophthalmol. Vis. Sci. 50 (8): 3763–70. doi:10.1167/iovs.08-2815.PMID 19264889.
- Lee BS, Margolin SB, Nowak RA (January 1998). “Pirfenidone: a novel pharmacological agent that inhibits leiomyoma cell proliferation and collagen production”. J. Clin. Endocrinol. Metab. 83 (1): 219–23. doi:10.1210/jc.83.1.219. PMID 9435445.
- Ozes ON, Blatt LM (2006). “Development of a high throughput collagen assay using a cellular model of idiopathic pulmonary fibrosis”. Chest 130: 230S.doi:10.1378/chest.130.4_meetingabstracts.230s-a.
- Sulfab M (2007). “The effects of pirfenidone and IFN-inducible T-cell alpha chemoattractant (ITAC) on transforming-growth factor-beta 1-mediated synthesis of extracellular matrix proteins in endothelial cells”. Am J Respir Crit Care Med 175: A730.
- Nakayama S, Mukae H, Sakamoto N, Kakugawa T, Yoshioka S, Soda H, Oku H, Urata Y, Kondo T, Kubota H, Nagata K, Kohno S (January 2008). “Pirfenidone inhibits the expression of HSP47 in TGF-beta1-stimulated human lung fibroblasts”. Life Sci. 82 (3–4): 210–7. doi:10.1016/j.lfs.2007.11.003. PMID 18093617.
- Phillips R, Wang T, Blatt LM, Seiwert S (2005). “Pirfenidone mediates differential effects on lipopolysaccharide-induced cytokine expression in human peripheral mononuclear cells”. Chest 128. doi:10.1378/chest.128.4_meetingabstracts.169s-a.
- Grattendick KJ, Nakashima JM, Feng L, Giri SN, Margolin SB (May 2008). “Effects of three anti-TNF-alpha drugs: etanercept, infliximab and pirfenidone on release of TNF-alpha in medium and TNF-alpha associated with the cell in vitro”. Int. Immunopharmacol.8 (5): 679–87. doi:10.1016/j.intimp.2008.01.013. PMID 18387510.
- ^ Jump up to:a b c Kakugawa T, Mukae H, Hayashi T, Ishii H, Abe K, Fujii T, Oku H, Miyazaki M, Kadota J, Kohno S (July 2004). “Pirfenidone attenuates expression of HSP47 in murine bleomycin-induced pulmonary fibrosis”. Eur. Respir. J. 24 (1): 57–65.doi:10.1183/09031936.04.00120803. PMID 15293605.
- Oku H, Shimizu T, Kawabata T, Nagira M, Hikita I, Ueyama A, Matsushima S, Torii M, Arimura A (August 2008). “Antifibrotic action of pirfenidone and prednisolone: different effects on pulmonary cytokines and growth factors in bleomycin-induced murine pulmonary fibrosis”. Eur. J. Pharmacol. 590 (1–3): 400–8.doi:10.1016/j.ejphar.2008.06.046. PMID 18598692.
- Card JW, Racz WJ, Brien JF, Margolin SB, Massey TE (September 2003). “Differential effects of pirfenidone on acute pulmonary injury and ensuing fibrosis in the hamster model of amiodarone-induced pulmonary toxicity”. Toxicol. Sci. 75 (1): 169–80.doi:10.1093/toxsci/kfg167. PMID 12832656.
- Liu H, Drew P, Gaugler AC, Cheng Y, Visner GA (June 2005). “Pirfenidone inhibits lung allograft fibrosis through L-arginine-arginase pathway”. Am. J. Transplant. 5 (6): 1256–63.doi:10.1111/j.1600-6143.2005.00876.x. PMID 15888029.
- Hirano A, Kanehiro A, Ono K, Ito W, Yoshida A, Okada C, Nakashima H, Tanimoto Y, Kataoka M, Gelfand EW, Tanimoto M (September 2006). “Pirfenidone modulates airway responsiveness, inflammation, and remodeling after repeated challenge”. Am. J. Respir. Cell Mol. Biol. 35 (3): 366–77. doi:10.1165/rcmb.2005-0452OC.PMC 2643289. PMID 16675785.
- Iyer SN, Wild JS, Schiedt MJ, Hyde DM, Margolin SB, Giri SN (June 1995). “Dietary intake of pirfenidone ameliorates bleomycin-induced lung fibrosis in hamsters”. J. Lab. Clin. Med. 125 (6): 779–85. PMID 7539478.
- Iyer SN, Margolin SB, Hyde DM, Giri SN (1998). “Lung fibrosis is ameliorated by pirfenidone fed in diet after the second dose in a three-dose bleomycin-hamster model”.Exp. Lung Res. 24 (1): 119–32. doi:10.3109/01902149809046058. PMID 9457473.
- Iyer SN, Gurujeyalakshmi G, Giri SN (April 1999). “Effects of pirfenidone on procollagen gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis”.J. Pharmacol. Exp. Ther. 289 (1): 211–8. PMID 10087006.
- Jump up^ Lee KW, Everett TH, Rahmutula D, Guerra JM, Wilson E, Ding C, Olgin JE (October 2006). “Pirfenidone prevents the development of a vulnerable substrate for atrial fibrillation in a canine model of heart failure”. Circulation 114 (16): 1703–12.doi:10.1161/CIRCULATIONAHA.106.624320. PMC 2129103. PMID 17030685.
- Nguyen DT, Ding C, Wilson E, Marcus GM, Olgin JE (October 2010). “Pirfenidone mitigates left ventricular fibrosis and dysfunction after myocardial infarction and reduces arrhythmias”. Heart Rhythm 7 (10): 1438–45. doi:10.1016/j.hrthm.2010.04.030.PMID 20433946.
- Mirkovic S, Seymour AM, Fenning A, Strachan A, Margolin SB, Taylor SM, Brown L (February 2002). “Attenuation of cardiac fibrosis by pirfenidone and amiloride in DOCA-salt hypertensive rats”. Br. J. Pharmacol. 135 (4): 961–8.doi:10.1038/sj.bjp.0704539. PMC 1573203. PMID 11861324.
- Jump up^ Shimizu T, Kuroda T, Hata S, Fukagawa M, Margolin SB, Kurokawa K (July 1998). “Pirfenidone improves renal function and fibrosis in the post-obstructed kidney”. Kidney Int. 54 (1): 99–109. doi:10.1046/j.1523-1755.1998.00XXX.x. PMID 9648068.
- Jump up^ Takakuta K, Fujimori A, Chikanishi T, Tanokura A, Iwatsuki Y, Yamamoto M, Nakajima H, Okada M, Itoh H (March 2010). “Renoprotective properties of pirfenidone in subtotally nephrectomized rats”. Eur. J. Pharmacol. 629 (1–3): 118–24.doi:10.1016/j.ejphar.2009.12.011. PMID 20006961.
- Jump up^ Salazar-Montes A, Ruiz-Corro L, López-Reyes A, Castrejón-Gómez E, Armendáriz-Borunda J (October 2008). “Potent antioxidant role of pirfenidone in experimental cirrhosis”. Eur. J. Pharmacol. 595 (1–3): 69–77. doi:10.1016/j.ejphar.2008.06.110.PMID 18652820.
- Jump up^ García L, Hernández I, Sandoval A, Salazar A, Garcia J, Vera J, Grijalva G, Muriel P, Margolin S, Armendariz-Borunda J (December 2002). “Pirfenidone effectively reverses experimental liver fibrosis”. J. Hepatol. 37 (6): 797–805. doi:10.1016/S0168-8278(02)00272-6. PMID 12445421.
- ^ Jump up to:a b c d e f g h i j k l “Esbriet 267 mg hard capsules”. Summary of product characteristics. European Medicines Agency.
- ^ Jump up to:a b Taniguchi H, Ebina M, Kondoh Y, et al. (April 2010). “Pirfenidone in idiopathic pulmonary fibrosis”. Eur. Respir. J. 35 (4): 821–9. doi:10.1183/09031936.00005209.PMID 19996196.
- ^ Jump up to:a b c d Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, King TE, Lancaster L, Sahn SA, Szwarcberg J, Valeyre D, du Bois RM (May 2011). “Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials”. Lancet 377 (9779): 1760–9. doi:10.1016/S0140-6736(11)60405-4.PMID 21571362.
- Jump up^ http://www.intermune.com/pirfenidone.
- ^ Jump up to:a b Spagnolo P, Del Giovane C, Luppi F, Cerri S, Balduzzi S, Walters EH, D’Amico R, Richeldi L (2010). “Non-steroid agents for idiopathic pulmonary fibrosis”. Cochrane Database Syst Rev (9): CD003134. doi:10.1002/14651858.CD003134.pub2.PMID 20824834.
- Jump up^ http://www.kitoscell.com
- Jump up^ A controlled clinical trial with pirfenidone in the treatment of pathological skin scarring caused by burns in pediatric patients, annals of plastic surgery, volume 68, number 1, january 2012
- Jump up^ The multifaceted role of pirfenidone and its novel target. Macias-Barragan et al. Fibrogenesis 6 Tissue Repair 2010, 3:16
- Pirfenidone effectively reverses experimental liver fibrosis. Journal of Hepatology, 37 (2002) 797-805
- Frieden J (2010-05-10). “FDA Nixes Pirfenidone for Now, Wants New Trial”. MedPage Today.
- “Efficacy and Safety of Pirfenidone in Patients With Idiopathic Pulmonary Fibrosis (IPF)”. NCT01366209. U.S. National Institutes of Health: ClinicalTrials.gov.
MORE MORE…………..
Cottin, Vincent; Wijsenbeek, M.; Bonella, F.; Vancheri, C.Slowing progression of idiopathic pulmonary fibrosis with pirfenidone: from clinical trials to real-life experience.Clinical Investigation (London, United Kingdom) (2014), 4(4), 313-326.
Zhang, Kang.Application of pirfenidone in manuf. of anti-angiogenic drugs.Faming Zhuanli Shenqing (2014), CN 103800325 A 20140521.
Ma, Zhen; Pan, Youlu; Huang, Wenhai; Yang, Yewei; Wang, Zunyuan; Li, Qin; Zhao, Yin; Zhang, Xinyue; Shen, Zhengrong.Synthesis and biological evaluation of the pirfenidone derivatives as antifibrotic agents.Bioorganic & Medicinal Chemistry Letters (2014), 24(1), 220-223.
Ramachandran Radhakrishnan, Michael Cyr, Sabine M. Pyles.Method for synthesizing pirfenidone.US Patent Number: US8519140 B2 , Also published as:CA2764043A1, CN102482255A, EP2440543A2, EP2440543A4, US20110003863, US20120016133, US20130345430, WO2010141600A2, WO2010141600A3,Publication date: Aug 27, 2013.Priority date:Jun 3, 2009.Original Assignee: Intermune, Inc.
Li, Fa and Wang, Ping,A new method for preparation of pirfenidone, Anhui Huagong, 38(4), 27, 31; 2012
Du, Zhenxin et al,Preparation of pirfenidone, Faming Zhuanli Shenqing, CN102558040, 11 Jul 2012
一种吡非尼酮的制备方法,申请号:CN 201110447487,公开(公告)号:CN102558040 A,
Zhang, Chengzhi and Sommers, Andreas, Substituted n-aryl pyridinones, PCT Int. Appl., WO2012122165, 13 Sep 2012
Hu, Gaoyun et al,1-(Substituted aryl)-5-((substituted arylamino)methyl)pyridin-2(1H)-one useful in the treatment of cancer and its preparation, Faming Zhuanli Shenqing, CN102241625, 16 Nov 2011
Qiang, Jianhua and Shi, Wei,A process for preparing pirfenidone,Faming Zhuanli Shenqing, CN101891676, 24 Nov 2010
Radhakrishnan, Ramachadran et al,Process for preparation of pirfenidone from bromobenzene and 5-methyl-2-pyridone in the presence of cuprous oxide and an organic solvent. PCT Int. Appl., WO2010141600, 09 Dec 2010
Gant, Thomas G. and Sarshar, Sepehr.Preparation of substituted N-aryl pyridinones as fibrotic inhibitors, PCT Int. Appl., WO2008157786, 24 Dec 2008
Magana Castro, Jose Agustin Rogelio et al,New process of synthesis for obtaining 5-methyl-1-phenyl-2-(H)-pyridone, pharmaceutical compositions and use thereof as cytoprotective and dermatological agent in topical applications,PCT Int. Appl., WO2008147170, 04 Dec 2008
Ma, Zhen et al,Synthesis of pirfenidone,Zhongguo Yiyao Gongye Zazhi, 37(6), 372-373; 2006
Ma, Zhen and Wang, Zunyuan,Process for preparation of pirfenidone as antifibrotic agent, Faming Zhuanli Shenqing Gongkai Shuomingshu, CN1817862, 16 Aug 2006
一种抗纤维化药物吡非尼酮的制备方法,申请号:200610049852.5,申请日:2006.03.15,公开(公告)号:CN1817862,
Tao, Lijian et al,Preparation of pyridone derivatives for treatment of fibrosis, Faming Zhuanli Shenqing, CN1386737, 25 Dec 2002
Taniguchi, Tomoko et al, Process for preparation of 5-methyl-1-phenyl-2(1H)-pyridinone by phenylation of 5-methyl-2(1H)-pyridinone with bromobenzene, PCT Int. Appl., WO2003014087, 20 Feb 2003
Talmadge King et al. A Phase 3 Trial of Pirfenidone in Patients with Idiopathic Pulmonary Fibrosis. NEJM May 18, 2014. DOI: 10.1056/NEJMoa1402582.
Raghu G, et al “Treatment of idiopathic pulmonary fibrosis with ambrisentan: A parallel, randomized trial” Ann Intern Med 2013; DOI: 10.7326/0003-4819-158-9-201305070-00003.
Luca Richeldi et al. Efficacy and Safety of Nintedanib in Idiopathic Pulmonary Fibrosis,N Engl J Med 2014; 370:2071-2082 , May 29, 2014DOI: 10.1056/NEJMoa1402584 (INPULSIS-1 and INPULSIS-2 ClinicalTrials.gov numbers, NCT01335464 and NCT01335477.)
 Nalmefene
Nalmefene





















 MOSHE ARKIN
MOSHE ARKIN