Organic spectroscopy should be brushed up and you get confidence
read my blog
ORGANIC SPECTROSCOPY INTERNATIONAL is the blog
Organic chemists from Industry and academics to interact on Spectroscopy techniques for Organic compounds ie NMR, MASS, IR, UV Etc. email me ……….. amcrasto@gmail.com
http://orgspectroscopyint.blogspot.in/ is the link
 amcrasto@gmail.com
amcrasto@gmail.com
Oleanolic acid spectral data and interpretation
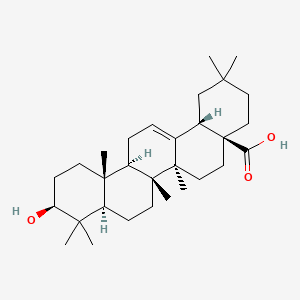
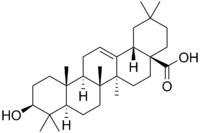 Oleanolic acid
Oleanolic acid(4aS,6aR,6aS,6bR,8aR,10S,12aR,14bS)-10-hydroxy-2,2,6a,6b,9,9,12a-heptamethyl-1,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylic acid
http://orgspectroscopyint.blogspot.in/2014/08/oleanolic-acid-spectral-data-and.html

Ursolic acid [(3b)-3-Hydroxyurs-12-en-28-oic acid] rarely occurs without its isomer oleanolic acid [(3b)-3-Hydroxyolean-12-en-28-oic acid] They may occur in their free acid form, as shown in Figure 1, or as aglycones for triterpenoid saponins which are comprised of a triterpenoid aglycone linked to one or more sugar moieties. Ursolic and oleanolic acids are similar in pharmacological activity
A pentacyclic triterpene that occurs widely in many PLANTS as the free acid or the aglycone for many SAPONINS. It is biosynthesized from lupane. It can rearrange to the isomer, ursolic acid, or be oxidized to taraxasterol and amyrin.
MS
EIMS m/z (rel. int.) 456 [M]+ (5), 412 (3), 248 (100), 203 (50), 167 (25), 44 (51)
IR KBR
(KBr) 3500, 2950, 2850, 1715; 1H-NMR (250 MHz, pyridine-d5) δ: 5.49 (1H, s, H-12), 3.47 (1H, t, J = 8.0 Hz, H-3), 3.30 (1H, m, H-18), 1.12 (3H, s, CH3-27), 0.96 (3H, s, CH3-30), 0.91 (3H, s, CH3-25), 0.89 (3H, s, CH3-23), 0.87 (3H, s, CH3-24), 0.75 (3H, s, CH3-26)
http://orgspectroscopyint.blogspot.in/2014/08/oleanolic-acid-spectral-data-and.html
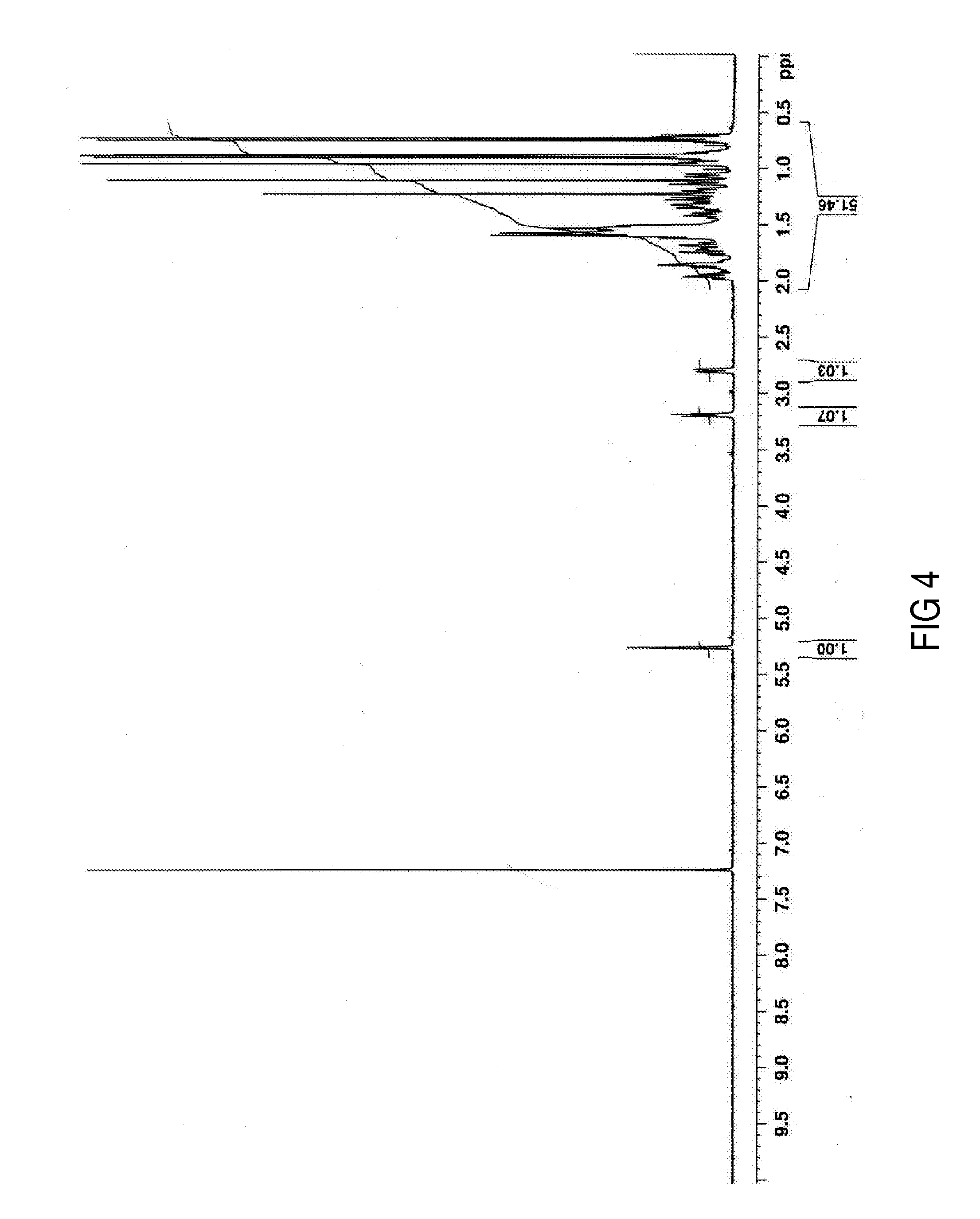
1H NMR
(250 MHz, pyridine-d5)δ: 5.49 (1H, s, H-12), 3.47 (1H, t, J = 8.0 Hz, H-3), 3.30 (1H, m, H-18), 1.12 (3H, s, CH3-27), 0.96 (3H, s, CH3-30), 0.91 (3H, s, CH3-25), 0.89 (3H, s, CH3-23), 0.87 (3H, s, CH3-24), 0.75 (3H, s, CH3-26) |

13 C NMR
(63 MHz, pyridine-d5) δ: 180.2 (C-28), 144.8 (C-13), 122.5 (C-12), 78.0 (C-3), 55.7 (C-5), 48.0 (C-9), 46.6 (C-8, 17), 42.1 (C-14), 39.7 (C-4), 39.4 (C-1), 37.3 (C-10), 33.2 (C-7), 32.9 (C-29), 32.4 (C-21), 30.9 (C-20), 28.7 (C-23), 27.2 (C-2), 26.9 (C-15), 26.1 (C-30), 23.7 (C-11), 23.6 (C-16), 18.7 (C-6), 17.4 (C-26), 16.5 (C-24), 15.5 (C-25) |
http://orgspectroscopyint.blogspot.in/2014/08/oleanolic-acid-spectral-data-and.html

http://www.google.com/patents/US20120237629
http://orgspectroscopyint.blogspot.in/2014/08/oleanolic-acid-spectral-data-and.html



EXAMPLE 2 Extraction and Isolation of Oleanolic Acid (9) and Maslinic Acid (10) from Cloves

THANKS AND REGARD’S
DR ANTHONY MELVIN CRASTO Ph.D
web link

New Drug Approvals, ALL ABOUT DRUGS, WORLD DRUG TRACKER
MEDICINAL CHEM INTERNATIONAL, DRUG SYN INTERNATIONAL
SCALEUP OF DRUGS, ALL FOR DRUGS ON WEB,
MY CHINA, VIETNAM AND JAPAN BLOGS
ICELAND, RUSSIA, ARAB
BOBRDOBR, BLAND ICELAND, 100zakladok, adfty
GROUPS
you can post articles and will be administered by me on the google group which is very popular across the world
OPD GROUPSPACES, SCOOP OCI, organic-process-development GOOGLE, TVINX, MENDELEY WDT,SCIPEOPLE OPD, EPERNICUS OPD, SYNTHETIC ORGANIC CHEMISTRYLinkedIn group, DIIGO OPD,LINKEDIN OPD, WDT LINKEDIN, WDTI ZING

![]()
Filed under: spectroscopy Tagged: NMR, oleanolic acid, ORGANIC SPECTROSCOPY INTERNATIONAL
