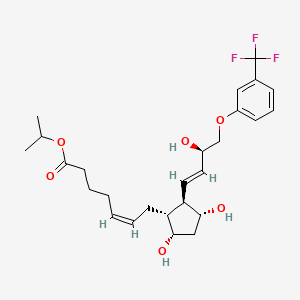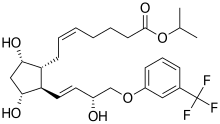
Travoprost
cas 157283-68-6
[1R-[lα(Z),2β(lE,3R*),3α,5α]]-7-[3,5-Dihydroxy-2-[3-hydroxy-4-[3-(trifluoromethyl)phenoxy]-1 -butenyl]cyclopentyl]-5-heptenoic acid, 1 -methylethylester
(+)-16-m-trifluoromethylphenoxy tetranor Prostaglandin F2α isopropyl ester; (+)-Fluprostenol ispopropyl ester
(+)-(5Z,9α,1α,13E,15R)-trihydroxy-16-(3-(trifluoromethyl)phenoxy)-17,18,19,20-tetranor-prosta-5,13-dien-1-oic acid, isopropyl ester
(+) – Fluprostenol isopropyl ester,

Ophthalmic solution used for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension who are intolerant of other intraocular pressure lowering medications or insufficiently responsive (failed to achieve target IOP determined after multiple measurements over time) to another intraocular pressure lowering medication.
Travoprost free acid is a selective FP prostanoid receptor agonist and is believed to reduce intraocular pressure by increasing the drainage of aqueous humor, which is done primarily through increased uveoscleral outflow and to a lesser extent, trabecular outflow facility.
Travoprost, an isopropyl ester prodrug, is a synthetic prostaglandin F2 alpha analogue that is rapidly hydrolyzed by esterases in the cornea to its biologically active free acid. The travoporst free acid is potent and highly selective for the FP prostanoid receptor.
Travoprost ophthalmic solution is a topical medication used for controlling the progression of glaucoma or ocular hypertension, by reducing intraocular pressure. It is a synthetic prostaglandin analog (or more specifically, an analog of prostaglandin F2α)[1][2] that works by increasing the outflow of aqueous fluid from the eyes.[3] It is also known by the brand names of Travatan and Travatan Z, manufactured by Alcon, and Travo-Z, manufactured by Micro Labs.
Travoprost is a synthetic prostaglandin F analogue. Its chemical name is [1R-[lα(Z),2β(lE,3R*),3α,5α]]-7-[3,5-Dihydroxy-2-[3-hydroxy-4-[3-(trifluoromethyl)phenoxy]-1 -butenyl]cyclopentyl]-5-heptenoic acid, 1 -methylethylester. It has a molecular formula of C26H35F3O6 and a molecular weight of 500.55. The chemical structure of travoprost is:

Country |
Patent Number |
Approved |
Expires (estimated) |
|
| Canada | 2181172 | 2003-04-29 | 2015-12-19 | |
| Canada | 2129287 | 2002-05-14 | 2014-08-02 | |
| United States | 5631287 | 1994-12-22 | 2014-12-22 | |
| United States | 6503497 | 1995-05-06 | 2012-05-06 |
|
7-25-2012
|
TOPICAL APPLICATION OF TRAVOPROST FOR COMBATING HAIR LOSS
|
|
|
12-28-2011
|
Stable prostaglandin-containing compositions
|
|
|
7-22-2011
|
IMPROVED PROCESS FOR THE PRODUCTION OF BIMATOPROST
|
|
|
6-3-2011
|
Process for the Preparation of Prostaglandin Analogues and Intermediates Thereof
|
|
|
9-17-2010
|
Compositions and Methods for Reducing Body Fat
|
|
|
5-28-2010
|
COMPLEXES OF PROSTAGLANDIN DERIVATIVES AND MONOSUBSTITUTED, CHARGED BETA-CYCLODEXTRINS
|
|
|
4-30-2010
|
AMINO ACID SALTS OF PROSTAGLANDINS
|
|
|
4-30-2010
|
AMINO ACID SALTS OF PROSTAGLANDINS
|
|
|
2-24-2010
|
Compositions and methods for reducing body fat
|
|
|
1-15-2010
|
Process for the Production of Prostaglandins and Prostaglandin Analogs
|
|
4-3-2009
|
METHOD FOR SCREENING OF PROSTAGLANDIN COMPOUNDS COMPRISING AN OPTIMAL FORMULATION FOR THE ENHANCEMENT OF HAIR GROWTH AND THE STIMULATION OF FOLLICULAR ANAGEN AND FORMULATIONS RESULTING THEREFROM
|
|
|
3-9-2005
|
9,11-cycloendoperoxide pro-drugs of prostaglandin analogues for treatment of ocular hypertension and glaucoma
|
|
|
10-8-2004
|
Use of cloprostenol and fluprostenol analogues to treat glaucoma and ocular hypertension
|
|
|
4-21-2004
|
Use of cloprostenol and fluprostenol analogues to treat glaucoma and ocular hypertension
|
Side effects
Possible side effects of this medication are:
- May cause blurred vision
- May cause eyelid redness
- May permanently darken eyelashes
- May cause eye discomfort
- May eventually cause permanent darkening of the iris to brown (heterochromia)
- May cause a temporary burning sensation during use
- May cause thickening of the eyelashes
- May cause inflammation of the prostate gland, restricting urine flow (BPH)
 |
|
| Systematic (IUPAC) name | |
|---|---|
| propan-2-yl 7-[3,5-dihydroxy-2-[3-hydroxy-4-[3-(trifluoromethyl) phenoxy]-but-1-enyl]-cyclopentyl]hept-5-enoate |
|
| Clinical data | |
| Trade names | Travatan |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a602027 |
| Pregnancy cat. | C US |
| Legal status | Rx only (US) |
| Routes | Topical (eye drops) |
| Identifiers | |
| CAS number | 157283-68-6  |
| ATC code | S01EE04 |
| PubChem | CID 5282226 |
| DrugBank | DB00287 |
| ChemSpider | 4445407  |
| UNII | WJ68R08KX9  |
| Chemical data | |
| Formula | C26H35F3O6 |
| Mol. mass | 500.548 g/mol |


The condensation of 2- [3- (trifluoromethyl) phenoxy] acetyl chloride (I) with methylphosphonic acid dimethyl ester (II) by means of BuLi in THF gives 2-oxo-3- [3- (trifluoromethyl) phenoxy] propylphosphonic acid dimethyl ester (III), which is condensed with the known bicyclic aldehyde (IV) by means of BuLi in dimethoxyethane, yielding the unsaturated ketone (V). The reduction of (V) with zinc borohydride in dimethoxyethane affords the unsaturated alcohol (VI), which is treated with K2CO3 to give a diastereomeric mixture of unsaturated diols, resolved by chromatography to yield the chiral unsaturated diol (VII). The protection of (VII) with dihydropyran and TsOH in dichloromethane provides the bis (tetrahydropyranyl) ether (VIII), which by reduction of the lactone ring with diisobutylaluminum hydride in THF gives the lactol (IX). The condensation of (IX) with the phosphonium bromide (X) by means of NaH in DMSO yields the prostenoic acid (XI), which is esterified with isopropyl iodide and 1,8-diazabicyclo [5.4.0] undec-7-ene (DBU) in acetone to afford the corresponding isopropyl ester (XII). Finally, this compound is deprotected with acetic acid in hot THF / water.
http://www.chemdrug.com/databases/8_0_qkvreurfepijmjcf.html
…………………………………………..
Org Process Res Dev2002,6, (2): 138
http://pubs.acs.org/doi/abs/10.1021/op010097p

A commercial synthesis of the antiglaucoma agent, travoprost 2, is described. A total of 22 synthetic steps are required to provide the single enantiomer prostanoid, with the longest linear sequence being 16 steps from 3-hydroxybenzotrifluoride. The route is based upon a cuprate-mediated coupling of the single enantiomer vinyl iodide 13 and the tricyclic ketone 5, of high stereochemical purity, to yield the single isomer bicyclic ketone 15. A Baeyer−Villiger oxidation provides the lactone 16 as a crystalline solid, thus limiting the need for chromatographic purification. DIBAL-H reduction, Wittig reaction, esterification, and silyl group deprotection complete the synthesis of travoprost.
(5Z,13E)-(9S,11R,15R)-9,11,15-Trihydroxy-16-(m-trifluoromethylphenoxy-17,18,19,20-tetranor-5,13-prostadienoic Acid, Isopropyl Ester (2).
The silyl-protected compound (20a+b) (202 g, 277 mmol) ………..DELETED……………………………………… All relevant fractions were combined and concentrated to give the title compound 2 (97 g, 70%) as a colourless oil,  +14.6 (c 1.0, CH2Cl2); IR νmax (film) 3374 and 1727 cm-1; 1H NMR (400 MHz, CDCl3) δ 7.39 (1H, t, J = 8), 7.22 (1H, d, J = 8), 7.15 (1H, s), 7.08 (1H, d, J = 8), 5.70 (2H, m), 5.40 (2H, m), 4.98 (1H, heptet, J = 6.5), 4.52 (1H, m), 4.18 (1H, m), 3.97 (3H, m), 3.25 (2H, br s), 2.60 (1H, br s), 2.38 (1H, m), 2.30−1.96 (7H, m), 1.76 (1H, dd, J = 16, 4), 1.65 (2H, quintet, J = 7), 1.55 (1H, m), and 1.20 (6H, d, J = 6); 13C NMR (100 MHz, CDCl3) δ 173.57, 158.67, 135.45, 131.87 (q, J = 32), 130.02, 129.85, 129.75, 128.93, 123.89 (q, J = 270), 118.06, 117.82, 111.48, 77.77, 72.70, 71.99, 70.86, 67.72, 55.82, 50.24, 42.84, 34.00, 26.60, 25.48, 24.83, and 21.81; m/z (CI) 501 (MH+, 21), 321 (34), 303 (44), and 249 (100).
+14.6 (c 1.0, CH2Cl2); IR νmax (film) 3374 and 1727 cm-1; 1H NMR (400 MHz, CDCl3) δ 7.39 (1H, t, J = 8), 7.22 (1H, d, J = 8), 7.15 (1H, s), 7.08 (1H, d, J = 8), 5.70 (2H, m), 5.40 (2H, m), 4.98 (1H, heptet, J = 6.5), 4.52 (1H, m), 4.18 (1H, m), 3.97 (3H, m), 3.25 (2H, br s), 2.60 (1H, br s), 2.38 (1H, m), 2.30−1.96 (7H, m), 1.76 (1H, dd, J = 16, 4), 1.65 (2H, quintet, J = 7), 1.55 (1H, m), and 1.20 (6H, d, J = 6); 13C NMR (100 MHz, CDCl3) δ 173.57, 158.67, 135.45, 131.87 (q, J = 32), 130.02, 129.85, 129.75, 128.93, 123.89 (q, J = 270), 118.06, 117.82, 111.48, 77.77, 72.70, 71.99, 70.86, 67.72, 55.82, 50.24, 42.84, 34.00, 26.60, 25.48, 24.83, and 21.81; m/z (CI) 501 (MH+, 21), 321 (34), 303 (44), and 249 (100).
…………………………………………
http://www.google.com/patents/EP2495235A1?cl=en
……………………………
http://www.google.com/patents/EP2454227A1?cl=en

Example 2
Synthesis of Travoprost MTBE MTBE
C
7b
8b
9b-iso
Travoprost Scheme 4. Synthesis of Travoprost
References
- Alcon Laboratories, Inc. (September 2011). “TRAVATAN – travoprost solution”. DailyMed. Bethesda, MD: U.S. National Library of Medicine. Retrieved 2011-09-30.
- Alcon Laboratories, Inc. (September 2011). “TRAVATAN Z (travoprost) solution”. DailyMed. Bethesda, MD: U.S. National Library of Medicine. Retrieved 2011-09-30.
- AHFS Consumer Medication Information (2011-01-01). “Travoprost Ophthalmic”. MedlinePlus. Bethesda, MD: U.S. National Library of Medicine. Retrieved 2011-09-30.
More References:
Selective FP prostaglandin receptor agonist. Isopropyl ester of (+)-fluprostenol, q.v. General prepn (not claimed): J. W. Stjernschantz, EP 364417 (1989 to Pharmacia).
Large scale synthesis: L. T. Boulton et al., Org. Process Res. Dev. 6, 138 (2002).
Pharmacology: M. R. Hellberg et al., J. Ocul. Pharmacol. Ther. 17, 421 (2001).
LC/MS/MS determn in plasma: B. A. McCue et al., J. Pharm. Biomed. Anal. 28, 199 (2002). Ocular hypotensive effects in dogs: A. B. Carvalho et al., Vet. Ophthalmol. 9, 121 (2006).
Clinical trial in glaucoma or ocular hypertension: R. L. Fellman et al., Ophthalmology 109, 998 (2002); in combination with timolol: J. S. Schuman et al., Am. J. Ophthalmol. 140, 242-250 (2005).
- Ota T, Aihara M, Narumiya S, Araie M: The effects of prostaglandin analogues on IOP in prostanoid FP-receptor-deficient mice. Invest Ophthalmol Vis Sci. 2005 Nov;46(11):4159-63. PubMed: 16249494
- Thieme H, Schimmat C, Munzer G, Boxberger M, Fromm M, Pfeiffer N, Rosenthal R: Endothelin antagonism: effects of FP receptor agonists prostaglandin F2alpha and fluprostenol on trabecular meshwork contractility. Invest Ophthalmol Vis Sci. 2006 Mar;47(3):938-45. PubMed: 16505027
- Lim KS, Nau CB, O’Byrne MM, Hodge DO, Toris CB, McLaren JW, Johnson DH: Mechanism of action of bimatoprost, latanoprost, and travoprost in healthy subjects. A crossover study. Ophthalmology. 2008 May;115(5):790-795.e4. PubMed: 18452763
- Neacsu AM: [Receptors involved in the mechanism of action of topical prostaglandines] Oftalmologia. 2009;53(2):3-7. PubMed: 19697832
- Costagliola C, dell’Omo R, Romano MR, Rinaldi M, Zeppa L, Parmeggiani F: Pharmacotherapy of intraocular pressure – part II. Carbonic anhydrase inhibitors, prostaglandin analogues and prostamides. Expert Opin Pharmacother. 2009 Dec;10(17):2859-70. PubMed: 19929706
- Ferrari G, Scagliotti GV: Serum and urinary vascular endothelial growth factor levels in non-small cell lung cancer patients. Eur J Cancer. 1996 Dec;32A(13):2368-9. PubMed: 9038626
- Toris CB, Gabelt BT, Kaufman PL: Update on the mechanism of action of topical prostaglandins for intraocular pressure reduction. Surv Ophthalmol. 2008 Nov;53 Suppl1:S107-20. PubMed: 19038618
- Arranz-Marquez E, Teus MA: Prostanoids for the management of glaucoma. Expert Opin Drug Saf. 2008 Nov;7(6):801-8. PubMed: 18983226
- Chen X, Ji ZL, Chen YZ: TTD: Therapeutic Target Database. Nucleic Acids Res. 2002 Jan 1;30(1):412-5. PubMed: 11752352
Filed under: Uncategorized Tagged: travoprost