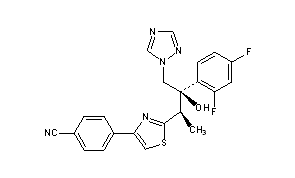
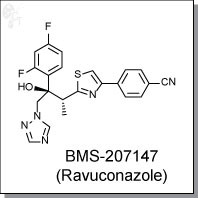
- BMS 207147
- ER 30346
- Ravuconazole
- UNII-95YH599JWV
4-[2-[(1R,2R)-2-(2,4-Difluorophenyl)-2-hydroxy-1-methyl-3-(1H-1,2,4-triazol-1-yl)propyl]-4-thiazolyl]benzonitrile


 |
|
| Systematic (IUPAC) name | |
|---|---|
| 4-[2-[(2R,3R)-3-(2,4-Difluorophenyl)-3-hydroxy-4-(1,2,4-triazol-1-yl)butan-2-yl]-1,3-thiazol-4-yl]benzonitrile | |
| Clinical data | |
| Legal status |
?
|
| Identifiers | |
| CAS number | 182760-06-1  |
| ATC code | None |
| PubChem | CID 467825 |
| ChemSpider | 411041  |
| NIAID ChemDB | 057176 |
| Chemical data | |
| Formula | C22H17F2N5OS |
| Mol. mass | 437.465086 g/mol |

ER-30346 is synthesized by thiazole ring formation of (2R, 3R) -3- (2,4-difluorophenyl) -3-hydroxy-2-methyl-4- (1H-1,2,4-triazol-1-yl ) thiobutanamide (I) and 4-bromoacetylbenzonitrile (II) by means of reflux in methanol. The thioamide (I) is obtained with excellent yield from a chiral nitrile (III) by heating with diethyl dithiophosphate in aqueous medium.



The nitrile (III), a chiral key intermediate of this synthesis, can be obtained by two different synthetic routes as follows: Route-b: The starting material of this route is methyl (S) -3-hydroxy-2-methylpropionate (VIII ), which contains one additional carbon between the hydroxyl group and the 2-position carbon of (R) -lactate, the starting material of route-a. The hydroxyl group of (VIII) is protected by triphenylmethyl group. Then, 2,4 -difluorophenyl moiety is introduced to give the ketone (X). Direct conversion of the ketone (X) to the oxirane (XIV) by dimethylsulfoxonium methylide, the same condition for compound (IV) in route-a, does not proceed. The oxirane (XIV) having desired stereochemistry is obtained via oxidation reaction. The ketone (X) is converted to the exomethylene (XI) by Wittig reaction. The stereoselective oxidation of (XI) is achieved by means of osmium tetroxide in the presence of 4-methylmorpholine N-oxide to give the diol (XII) in 58% yield after separation of its epimer by column chromatography. After methanesulfonylation of the primary alcohol of (XII), a triazole moiety is introduced and the triphenylmethyl group is deprotected. Then, the primary hydroxyl group of (XVI) is oxidized under Swern oxidation condition to give the aldehyde (XVII), which is converted to the chiral nitrile intermediate (III) by means of heating with hydroxylamine-O-sulfonic acid.![]()
The synthesis of (2S, 3S) -3- (2,4-difluorophenyl) -3-hydroxy-2-methyl-4- (1,2,4-triazol-1-yl) butyronitrile (XV), a key intermediate the synthesis of ER-30346 has been described: The tritylation of 3-hydroxy-2 (S) -methylpropionic acid methyl ester (I) with trityl chloride in hot pyridine gives the trityl ether (II), which is hydrolyzed with LiOH in H2O / THF / methanol yielding the free acid (III). The esterification of (III) with 2-mercaptopyridine (IV) by means of dicyclohexylcarbodiimide (DCC) in dichloromethane gives the thioester (V), which is treated with 2,4-difluorophenylmagnesium bromide (VI) in THF yielding the propiophenone (VII), which by treatment with methyltriphenylphosphonium bromide / NaH in THF is converted into the methylene derivative (VIII). The oxidation of (VIII) with OsO4 and N-methylmorpholine oxide in acetone affords, after column chromatography, the chiral diol (IX), which is monomesylated with mesyl chloride / triethylamine in dichlormethane giving the monoester (X). The reaction of (X) with 1,2,4-triazol (XI) and NaH in DMF yields (2R, 3S) -2- (2,4-difluorophenyl) -3-methyl-1- (1,2,4-triazol-1-yl) -4- (triphenylmethoxy) -2-butanol (XII), which is detritylated with p-toluenesulfonic acid in methanol affording the diol (XIII). The oxidation of (XIII) with oxalyl chloride / DMSO in dichloromethane gives the aldehyde (XIV), which is finally treated with hydroxylamine-O-sulfonic acid in water yielding the desired bytyronitrile intermediate (XV) already referenced.![]()
http://www.google.com/patents/WO2011042827A1?cl=en
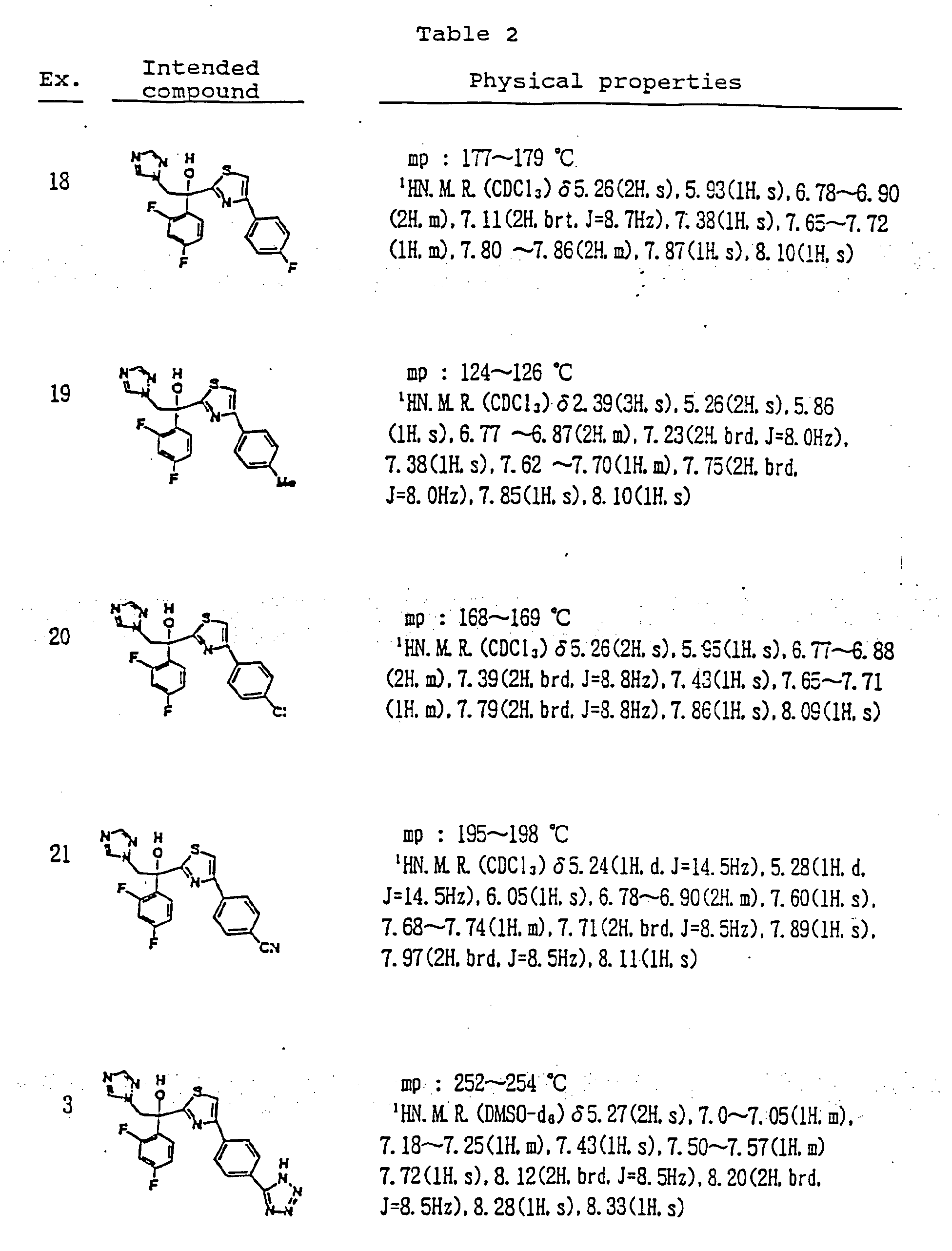
Example 1
(2R,3R)-3-i4-(4-cvanophenyl)thiazol-2-yl1-1 -(1 H-1 ,2,4-triazol-1 -yl)-2-(2,4-difluorophenyl)- butan-2-ol
To a solution of racemic 3-[4-(4-cyanophenyl)thiazol-2-yl]-1 -(1 H-1 ,2,4-triazol-1 -yl)-2-(2,4- difluorophenyl)-butan-2-ol (43.7 g) in acetone (800 ml) a solution of (1 R)-10- camphorsulfonic acid (23 g) in methanol (300 ml) was added and the mixture was heated under reflux until a clear solution was obtained. The solution was slowly cooled to rt, seeded with crystals of the title enantiomeric salt and let overnight. The solid was collected by filtration, washed with acetone and dried to provide (2R,3R)-3-[4-(4- cyanophenyl)thiazol-2-yl]-1 -(1 H-1 ,2,4-triazol-1 -yl)-2-(2,4-difluorophenyl)-butan-2-ol (1 R)- 10-camphorsulfonate as white solid. This crude salt was then taken up in methylenechloride (100 ml) and water (ca. 100 ml) and the mixture was basified with aqueous sodium hydroxide solution. The organic layer was separated and the aqueous phase washed twice with methylenechloride (50 ml) and combined. The organic phases were then washed twice with water (2×50 ml), dried with sodium sulfate, filtrated and the solvent removed under reduced pressure. The crude product was then mixed with isopropanol (ca. 150 ml), heated for 10 min, cooled to 0° C and stirred for ca. 2 hrs. The product was collected, washed with isopropanol and dried under reduced pressure to provide the enantiomerically pure title compound (17.5 g, 41 % yield, 99.1 % ee); m.p. 164-166° C; [a]=-30° (c=1 , methanol, 25° C); NMR (CDCI3): 1 .23(3H, d, J=8 Hz), 4.09(1 H, q, J=8Hz), 4.26(1 H, d, J=14Hz), 4.92(1 H, d, J=14Hz), 5.75(1 H, s), 6.75- 6.85(2H, m), 7.45-7.54(2H, m), 7.62(1 H, s), 7.69(1 H, s), 7.75(1 H, d, J=8Hz), 7.86(1 H, s), 8.03(1 H,d,J=8Hz). The analytical data were identical with published (US5648372 and Chem. Pharm. Bull. 1998, 46, 623-630).
…………………………
http://www.google.com/patents/WO1999045008A1?cl=en
Example 1
a) Preparation of (2R)-2′,5′-Difluoro-2-(3,4,5,6-tetrahydro-
2H-pyran-2-yloxy)-propiophenone A mixture of magnesium ( 7.25 g, 0.298 mol ) and iodine ( catalytic amount ) and l-bromo-2,5-difluorobenzene ( 20.0 g, 0.178 mol ) in THF ( 250ml ) was vigously stirred. The color of iodine was disappeared and the inner temperature rose up to 65°C. To this mixture was added additional l-bromo-2,5-difluorobenzene ( 30.0 g, 0.267 mol ) dropwise to maintain the inner temperature from 50 to 55°C over 45min. The resulting mixture was stirred at 55°C for 30min. then at r.t. for lhr. The – 21 -
mixture was cooled down to -5°C. To this mixture was added a solution of.4-[(2R)-2-(3,4,5,6-Tetrahydro-2H-pyran-2-yloxy)propionyl] morpholine ( 52.5 g, 0.216 mol ) in THF ( 150ml ) dropwise over 40min. And the resulting mixture was stirred at r.t. for 4hrs. The reaction mixture was cooled down to 5°C and saturated NH4C1 aq. ( 100ml ) was added carefully. The whole was diluted with H20 ( 600ml ) and extracted with EtOAc ( 400ml + 200ml x 2 ). The combined organic layer was dried over Na2S04 and concentrated in vacuo. The residue was chromatographed on silica gel ( n-hexane : EtOAc = 10 :1 ~ 5 : 1 ) to give (2R)-2′,5′- Difluoro-2-(3,4,5,6-tetrahydro-2H-pyran-2-yloxy)-propiophenone (47.3 g,
81 % ) as pale yellow syrup.
Physical form : colorless oil; FAB-MS: m/z 271(M+H)+; Η-NMR(CDCl;j): 1.42~1.90(9H,m),3.32~3.40(lHxl/2,m),3.69~3.77(lHxl/2,m),3.86~3.94 (lHxl/2,m),4.66(lHxl/2,t,J=3.6Hz),4.75(lHxl/2,t,J=3.6Hz),4.87(lHxl/2, q,J=6.6Hz),5.11(lHxl/2,q,J=6.9Hz),7.08~7.25(2H,m),7.49~7.55(lH,m).
b) Preparation of 2-(2,5-Difluorophenyl)-2-[(lR)-l-(3,4,5,6,- tetr ahy dro-2H-pyran-2-yloxy ) ethyl] oxir ane To a stirred mixture of NaH ( 60% in oil, 9.1g, 0.228mol ) in DMSO
(300ml ) was added portionwise trimethylsulfoxonium iodide ( 53.9g, 0.245 mol ) at the inner teperature with the range from 15°C to 18°C. over 20min. The ice bath was removed and the mixtuer was stirred at r.t. for 3hrs. The mixture was cooled down to 10°C. To this mixture was added a solution of (2R)-2′,5′-Difluoro-2-(3,4,5,6-tetrahydro-2H-pyran-2- yloxy)-propiophenone ( 47.3 g , 0.175 mol ) in DMSO (150ml ) dropwise over 20min. The resulting mixture was stirred at r.t. for 4hrs. The reaction mixture was poured into ice-water ( 800ml ). The whole was extracted with EtOAc ( 400ml + 200ml x 2 ). The combined organic layer was washed with brine, dried over Na2S04 and concentrated in vacuo.
The residue was chromatograkkphed on silicagel ( n-hexane : EtOAc = – 22 -
8 : 1 ~ 5 : 1 ) to give 2-(2,5-Difluorophenyl)-2-[(lR)-l-(3,4,5,6,- tetrahydro-2H-pyran-2-yloxy)ethyl]oxirane (48.3 g, 97 % ). Physical form : pale yellow syrup, EI-MS: m/z 284 (M)+ ; 1H-NMR(CDC13): 1.15(3Hxl/2,dd,J=6.6,1.3Hz), 1.24(3Hxl/2,dd, J=6.6,1.3Hz), 1.52-1.87 (6H,m),2.83~2,90(lH,m),3.07
(lHxl/2,d,J=5.3Hz),3.36(lHxl/2,d,J=5.6Hz), 3.48~3.56(lH,m),3.82~3.92 (lH,m),4.00~4.16(lH,m),4.73~4.92(lH,m), 6.96~7.02(lH,m),7.09~7.15 (lH,m).
c) Preparation of (3R)-2-(2,5-difluorophenyl)-3-(3,4,5,6- tetrahydro-2H-pyran-2-yloxy)-l-(lH-l,2,4-triazol-l-yl)-2-butanol
To a stirred suspension of NaH ( 60 % in oil, 21.0 g, 0.525 mol ) in DMF (300ml ) was added portionwise 1,2,4-triazole ( 43.3 g, 0.627 mol ) at the inner temperature from 2°C to 11°C over 30min. The resulting mixture was stirred at r.t. for l.δhrs. To this mixture was added a solution of 2-(2,5-Difluorophenyl)-2-[(lR)-l-(3,4,5,6-tetrahydro-2H- pyran-2-yloxy)ethyl]oxirane ( 48.3 g, 0.170 mol ) in DMF ( 50 ml ). The mixture was stirred at 60°C for lhr. and then at 65°C for 14hrs. The reaction mixture was cooled down to 10°C and then poured into ice- water (800 mL ). The resulting mixture was extracted with EtOAc
(400ml + 200ml x 2 ). The combined organic layer was dried over Na2S04 and concentrated in vacuo. The residue was chromatographed on silicagel ( n-hexane : EtOAc = 4 : 1 ~ 1 : 5 ) to give (3R)-2-(2,5- difluorophenyl)-3-(3,4,5,6-tetrahydro-2H-pyran-2-yloxy)-l-(lH-l,2,4- triazol-l-yl)-2-butanol ( 43.9 g, 73 % ) and recovered starting material
(13.2 g, 27 % ).
Physical form : colorless syrup ; FAB-MS: m/z 354 (M+H)+ ; Η- NMR(CDCl3): 1.00(3Hxl/2,d,J=6.6Hz),1.13(3Hxl/2,d,J=6.6Hz), 1.42~1.88(6H,m),3.38~3.60 (lH,m),3.80~4.00(lH,m),4.32~5.02(5H,m),6.83~6.99 (2H,m),7.14-7.21
(lH,m),7.73(lHxl/2,s),7.74(lHxl/2,s),7.92(lHxl/2,s),7.95(lHxl/2,s). – 23 -
d) Preparation of (2R,3R)-2-(2,5-difluorophenyl)-l-(lH-l,2,4- triazol-l-yl)-2,3-butanediol
A mixture of (3R)-2-(2,5-difluorophenyl)-3-(3,4,5,6-tetrahydro-2H- pyran-2-yloxy)-l-(lH-l,2,4-triazol-l-yl)-2-butanol ( 43.9 g, 0.124 mol ) and PPTS ( 15.6 g, 62.1 mmol ) in EtOH ( 400ml ) was stirred at 55°C for 5hrs. The mixture was was evaporated to remove solvent down to 100ml. The residue was poured into ice-aqueous NaHC03 ( 500ml ). The whole was extracted with EtOAc ( 400ml + 200ml x 2 ). The combined organic layer was dried over Na2S04 and concentrated in vacuo. The residue was chromatographed on silicagel (CH2C12 : MeOH = 20 : 1) to give (2R,3R)-2-(2,5-difluorophenyl)-l-(lH-l,2,4-triazol-l-yl)-2,3- butanediol (18.0 g, 54 % ). Physical form : colorless syrup ; FAB-MS: m/z 270 (M+H)’ ; ‘H- NMR(CDC13): 0.99(3H,d,J=6.6Hz),2.61(lH,d,J=10.6Hz), 4.31-4.36
(lH,m),4.79,4.88
(2H,ABq,J=14.5Hz),4.84(lH,s),6.84~6.99(2H,m),7.13~7.19(lH,m),7.84(l H,s),7.85(lH,s).
e) Preparation of (2R,3S)-2-(2,5-Difluorophenyl)-3-methyl-2-
[ ( 1H- 1 ,2,4-triazol-l -yl) -methyl] -oxir ane
To a cold ( 0°C ) and stirred solution of (2R,3R)-2-(2,5-difluorophenyl)- l-(lH-l,2,4-triazol-l-yl)-2,3-butanediol ( 35.0 g, 0.130 mol ) and triethylamine ( 54.8 ml, 0.393 mol ) in CH2C12 ( 500ml ) was added a mesylchloride ( 12.1 ml, 0.156 mol ) dropwise over 5min. The resulting mixture was stirred at r.t. for l.δhrs. The reaction mixture was poured into ice-water ( 300ml ). The resulting mixture was shaken well and the organic layer was separated. The aqueous layer was further extracted with CH2C12 ( 150ml x 2 ). All the organic layers were combined, dried over Na2SO4 and concentrated in vacuo to give mesylate ( 46.7 g ) as crude syrup. The obtained mesylate was dissolved in MeOH ( 500ml ) – 24 -
and the solution was cooled down to 0°C. To this solution was added 28% NaOMe methanol solution (29.0 ml ). The mixture was stirred at 0°C for 50min. The reaction mixture was evaporated to reduce the volume of the solvent down to 150 ml. The residue was poured into ice- water ( 300ml ). The resulting mixture was extracted with ethylacetate (300ml + 200ml x 2 ). The combined organic layer was dried over Na.,S0 and concentrated in vacuo. The residue was cromatographed on silicagel (hexane : EtOAc = 1 : 3 ) to give (2R,3S)-2-(2,5-Difluorophenyl)- 3-methyl-2-[(lH-l,2,4-triazol-l-yl)-methyl]-oxirane (30.3 g, 93 %).
Physical form : white solid ; FAB-MS : m z 252 (M+H)+ ; ]H- NMR(CDC13): 1.64(3H,d,J=5.6Hz),3.19(lH,q,J=5.6Hz),4.42,4.97 (2H,ABq,J=14.8Hz), 6.75~6.81(lH,m),6.89~7.01(2H,m),7.83(lH,s),7.98 UH,s).
f) Preparation of (2S,3R)-3-(2,5-Difluoro-phenyl)-3-hydroxy-
2-methyl-4-[l,2,4]triazol-l-yl-butyronitrile
A mixture of (2R,3S)-2-(2,5-Difluorophenyl)-3-methyl-2-[(lH-l,2,4- triazol-l-yl)-methyl]-oxirane ( 30.3 g, 0.121 mol ), trimethylsilylcyanide ( 65.0 ml ) and MgO ( 24.5 g ) in o-xylene ( 400 ml ) was stirred at 130°C for lOhrs. To this mixture was added additional trimethylsilylcyanide (20.0 ml ) and MgO ( 8.5 g ) and the resulting mixture was stirred at 130°C further for 6hrs. The reaction mixture was cooled down to r.t. The precipitate was filtered off and washed with CH2C12. The filtrate was concentrated in vacuo to give crude brown syrup.
This crude syrup was dissolved in THF ( 600ml ) and the solution was cooled down to 0°C. To this mixture was added 1.0 M tetra n- butylammoniumfluoride THF solution ( 133ml, 0.133 mol ) dropwise over 5min. The mixture was stirred at r.t. for 50min. The solvent was removed under reduced pressure down to 150ml. The residue was poured into ice-water ( 400ml ). The resulting mixture was extracted – 25 -
with EtOAc ( 300ml + 200ml x 2 ). The combined organic layer was dried over Na2SO4 and concentrated in vacuo. The residue was chromatographed on silicagel ( n-hexane : EtOAc = 1 : 3 ) to give (2S,3R)-3-(2,5-Difluoro-phenyl)-3-hydroxy-2-methyl-4-[l,2;4]triazol-l-yl- butyronitrile ( 30.5 g, 91 % ).
Physical form : colorless syrup ; FAB-MS : m/z 279 (M+H)+ ; Η- NMR(CDCl3): 1.19(3H,d,J=7.3Hz),3.33(lH,q,J=7.3Hz),4.82,5.00 (2H,ABq,J=13.9Hz), 5.56(lH,brs),6.89~7.04(2H,m),7.12~7.19(lH,m),7.85(lH,s),7.86(lH,s).
g) Preparation of (2R,3R)-3-(2,5-Difluoro-phenyl)-3-hydroxy-
2-methyl-4- [ 1 ,2,4] triazol-1 -ylthiob tyramide
A mixture of (2S,3R)-3-(2,5-Difluoro-phenyl)-3-hydroxy-2-methyl-4- [l,2,4]triazol-l-yl-butyronitrile ( 30-5 S> O.llOmol ), diethyldithio- phospate ( 235 ml ) and H2O ( 110 ml ) was stirre at 80°C for 2hrs. The reaction mixture was cooled down to r.t. n-Hexane ( 400ml ) and water (200 ml ) was added. The whole was shaken well and the aqueous layer was separated. The remaining organic layer was further extracted with H20 ( 100ml x 3 ). All the aqueous layer was combined. Cooled down to
0°C and neutralized and basified ( PH8 ) with NaHC03. This basic(PH8) aqueous layer was extracted with EtOAc ( 300ml + 100ml x 3 ). The combined organic layer was dried over Na2S04 and concentrated in vacuo to give dark brown syrup. By addition of CH2C12 ( 100ml ) to this crude syrup, precipitate was formed. The precipitate was filtered and washed with CH2C12-hexane ( 5 : 1 mixture ) to give (2R,3R)-3-(2,5-Difluoro-phenyl)-3-hydroxy-2-methyl-4-[l,2,4]triazol-l- ylthiobutyramide ( 19.2 g, 56 % ) as white powder. On the oter hand, the filtrate was concentrated in vacuo and the residue was chromatographed on silica gel ( Wako-gel C-300, CH2C12 : MeOH = 20 :
1 ) to give additional (2R,3R)-3-(2,5-Difluoro-phenyl)-3-hydroxy-2- – 26 -
methyl-4-[l,2,4]triazol-l-ylthiobutyramide ( 7.46 g, 22 % ) as pale brown amorphous powder.
Physical form : White solid ; FAB-MS : m/z 313 (M+H)+ ; ‘H-NMR (CDC13): 1.12(3H,d,J=7.3Hz),3.74(lH,q,J=7.3Hz), 4.55,5.12 (2H,ABq,J=14.5Hz), 5.84(lH,s),6.85~7.02(2H,m),7.15-7.22(lH,m),7.80
(1H,S),7.89(1H,S), 7.89(lH,brs),8.43(lH,brs).
h) Preparation of 4-{2-[(lR,2R)-2-(2,5-Difluoro-phenyl)-2- hydroxy-l-methyl-3-[l,2,4]triazol-l-yl-propyl]-thiazol-4-yl}- benzonitrile
A mixture of (2R,3R)-3-(2,5-Difluoro-phenyl)-3-hydroxy-2-methyl-4- [l,2,4]triazol-l-ylthiobutyramide ( 26.7 g, 85.4 mmol ) and a-bromo-4′- cyano-acetophenone ( 24.0 g, 0.107 mol ) in EtOH ( 500ml ) was refluxed for lhr. The reaction mixture was cooled down to r.t. And the solvent was removed under reduced pressure down to 150ml. The residue was poured into in to cold ( 0°C ) saturated NaHC03 aq. ( 400ml ). The resulting mixture was extracted with EtOAc ( 300ml + 150 ml x 2 ). The combined organic layer was washed with brine (200ml ), dried over Na2S04 and concentrated in vacuo. The residue was chromatographed on silica gel ( Wako-gel C-300, Hexane : EtOAc = 1 : 2 ) to give 4-{2-
[(lR,2R)-2-(2,5-Difluoro-phenyl)-2-hydroxy-l-methyl-3-[l,2,4]triazol-l- yl-propyl]-thiazol-4-yl}-benzonitrile ( 32.0 g, 86 % ).
Physical form : colorless heavy syrup ; ESI-MS : m/z 437 (M)+ ; ‘H-
NMR(CDCl3): 1.25(3H,d,J=7.3Hz),4.12(lH,q,J=7.3Hz),4.26,4.96 (2H,Abq,J=14.5Hz), 5.75(lH,s),6.89~7.07(2H,m),7.23~7.29(lH,m),7.65
(lH,s),7.71(lH,s),7.75,8.02 (4H,Abq,J=8.6Hz),7.85(lH,s). – 27 -
i) Preparation of 4-{4-[(tert-Butoxycarbonyl-methyl-amino)- acetoxy]-3,5-dimethyl-benzyl}-l-[(2R,3R)-3-[4-(4-cyano-phenyl)- thiazol-2-yl]-2-(2,5-difluoro-phenyl)-2-hydroxy-butyl]-lH- [l,2,4]triazol-4-ium bromide A mixture of 22.7mg of 4-{2-[(lR,2R)-2-(2,5-Difluoro-phenyl)-2-hydroxy- l-methyl-3-[l,2,4]triazol-l-yl-propyl]-thiazol-4-yl}-benzonitrile and 25.0mg of 4-tert-butoxycarbonyl-methyl-aminoacetoxy-3,5-dimethyl- benzyl bromide in CH3CN(1.5mL) was refluxed over 15hrs. The solvent was evaporated in vacuo and the residue was chromatographed on silica gel (Wakogel C-200, solvent:CH2Cl MeOH=10/l) to give 4-{4-[(tert-
Butoxycarbonyl-methyl-amino)-acetoxy]-3,5-dimethyl-benzyl}-l- [(2R,3R)-3-[4-(4-cyano-phenyl)-thiazol-2-yl]-2-(2,5-difluoro-phenyl)-2- hydroxy-butyl]-lH-[l,2,4]triazol-4-ium bromide (36.0mg, 84% as colorless heavy syrup) ; FAB-MS : m/z 743 (M-Br)’ ; Η-NMR(CDC1S): 1.23(3H,d,J=7.3Hz),
1.47(9H,s),2.14(6H,s),3.03(3H,s),4.15(lH,q,J=7.3Hz),4.25(2H,s), 4.98,5.16(2H,ABq,J=13.9Hz),5.39~5.54(2H,m),6.27(lH,s),6.89-7.07(4H, m),7.24~7.27(lH,m),7.58(lH,s),7.73,8.06(4H,ABq,J=8.58),8.07(lH,s),ll. 26 (lH,s).
j) Preparation of l-{(2R,3R)-3-[4-(4-cyano-phenyl)-thiazol-2-yl]- 2-(2,5-difluoro-phenyl)-2-hydroxy-butyl}-4-(3,5-dimethyl-4- methylaminoacetoxy-benzyl)-lH-[l,2,4]triazol-4-ium bromide To a solution of 36mg of 4-{4-[(tert-Butoxycarbonyl-methyl-amino)- acetoxy]-3,5-dimethyl-benzyl}-l-[(2R,3R)-3-[4-(4-cyano-phenyl)-thiazol-
2-yl]-2-(2,5-difluoro-phenyl)-2-hydroxy-butyl]-lH-[l,2,4]triazol-4-ium bromide in ethylacetate(2ml) was added dropwise 4N HC1 ethylacetate solution(lmL) and the mixture was stirred at r.t. for 4hrs.The precipitate was “filtered and washed with diethylether to give 1- {(2R,3R)-3-[4-(4-cyano-phenyl)-thiazol-2-yl]-2-(2,5-difluoro-phenyl)-2- hydroxy-butyl}-4-(3,5-dimethyl-4-methylaminoacetoxy-benzyl)-lH- – 28 -
[l,2,4]triazol-4-ium bromide (24.5mg, 74% as HC1 salt and as white solid) ;
FAB-MS : m/z 643 (M-Br)+ ; Η-NMR(DMSO-d): 1.19(3H,d,J=7.3Hz), 2.11(6H,s),2.64(3H,s),4.15(lH,q,J=7.3Hz),4.41(2H,s),4.74,5.04(2H,ABq,J =14.5Hz),5.40(2H,s),6.76(lH,brs),7.10(2H,s),7.20~7.38(2H,m), 7.94,8.21
(4H,ABq,J=8.25),8.45(lH,s),9.07(lH,s),9.50(lH,brs),10.17(lH,s).
………………………
http://www.google.co.in/patents/US5648372
OR
http://www.google.co.in/patents/EP1394142A1
COMPD 21
- Example 88:Preparation of a compound of the structural formula:
-
-
2-(2,4-Difluorophenyl)-3-thioamide-1-(1H-1,2,4-triazol-1-yl)-2-butanol (the raw material 2) (156 mg) was dissolved in EtOH (2 ml), and 2-bromo-4′-cyanoacetophenone (the raw material 3) (224 mg) was added to the solution, followed by heating and refluxing for 1 hour. The liquid reaction mixture was neutralized with a saturated aqueous solution of NaHCO3 and subjected to extraction with AcOEt. After the extract was washed with H2O and then a saturated aqueous solution of NaCl and dried over MgSO4, AcOEt was distilled out. The resultant residue was purified by chromatography on silica gel (SiO2: 20 g, eluted with CH2Cl2 and then with 1% solution of MeOH in CH2Cl2), and then crystallized from IPE, thereby obtaining the intended compound (109 mg). Physical properties of this compound are described below.
- mp:
- 196-197°C.
- NMR:
- δ solvent (CDCl3)
1.23(3H,d,J=8.0Hz), 4.09(1H,q,J=8.0Hz), 4.26(1H,d,J=14.3Hz), 4.92(1H,d,J=14.3Hz), 5.74(1H,s), 6.78-6.85(2H,m), 7.48-7.54(1H,m), 7.64(1H,s), 7.69(1H,s), 7.75(1H,d,J=8.1Hz), 7.85(1H,s), 8.03(1H,d,J=8.1Hz). - MS:
- MH+ = 438.
References
- National Cancer Institute. Ravuconazole in Preventing Fungal Infections in Patients Undergoing Allogeneic Stem Cell Transplantation. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000- [cited 2010 Feb 18]. Available from:http://clinicaltrials.gov/ct2/show/NCT00064311?term=ravuconazole&spons_ex=Y&rank=1 NLM Identifier: NCT00064311.
- The Aspergillus Website, Pasqualotto AC, Denning DW. Ravuconazole. Date accessed: 2010 Feb 18.
- Pasqualotto AC, Thiele KO, Goldani LZ (2010). “Novel triazole antifungal drugs: focus on isavuconazole, ravuconazole and albaconazole”. Curr Opin Investig Drugs 11 (2): 165–74. PMID 20112166.
- Pfaller, M. A.; Messer, S. A.; Hollis, R. J.; Jones, R. N.; Sentry Participants, Group (2002). “Antifungal Activities of Posaconazole, Ravuconazole, and Voriconazole Compared to Those of Itraconazole and Amphotericin B against 239 Clinical Isolates of Aspergillus spp. and Other Filamentous Fungi: Report from SENTRY Antimicrobial Surveillance Program, 2000″. Antimicrobial Agents and Chemotherapy46 (4): 1032. doi:10.1128/AAC.46.4.1032-1037.2002. PMC 127116. PMID 11897586.

Literature References:
Ergosterol biosynthesis inhibitor. Prepn (stereochemistry unspecified): T. Naito et al, EP 667346; eidem,US 5648372 (1995, 1997 both to Eisai); of optically acitve form: A. Tsuruoka et al., Chem. Pharm. Bull. 46, 623 (1998). Chiral synthesis: Y. Kaku et al., ibid. 1125.
In vitro comparative antifungal spectrum: J. C. Fung-Tomc et al., Antimicrob. Agents Chemother. 42, 313 1998. Antifungal activity in candidosis: K. V. Clemons, D. A. Stevens, ibid. 45, 3433 (2001); in aspergillosis: W. R. Kirkpatrick et al., J. Antimicrob. Chemother. 49, 353 (2002).
Clinical evaluation in onychomycosis: A. K. Gupta et al., J. Eur. Acad. Dermatol. Venereol. 19, 437 (2005).
Review of development and therapeutic potential: S. Arikan, J. H. Rex, Curr. Opin. Invest. Drugs 3, 555-561 (2002).
Extras you may need

http://www.google.com/patents/WO2011042827A1?cl=en
Scheme 1 :
The manufacturing process for Isavuconazole is similar: Since Isavuconazole differentiates from Ravuconazole by only another fluorine substitution on the aromatic ring (2,5- instead of 2,4-difluorophenyl), the identical synthesis has been used (US 6300353 from October 9, 2001 and Bioorg. & Med. Chem. Lett. 13, 191 (2003)). Consequently, also this manufacturing process, based on (R)-lactic acid, faces the same problems: to many steps, extremely low overall yield and in addition to US patent 6300353 claims even already known step as novel (claim 36).
Recent attempts to improve this concept as reported in WO 2007/062542 (Dec.1 , 2005), using less expensive, natural configured (S)-lactic acid, also failed: As already reported in US 6133485 and in US 2003/0236419, the second chiral center was formed from an optically active allyl alcohol prepared in a few steps from (S)-lactic acid. This allyl alcohol was subjected to Sharpless diastereoselective epoxidation providing first an opposite configured, epimeric epoxy alcohol which had to be then epimerized in an additional inversion step yielding finally the desired epoxy alcohol as the known precursor for Isavuconazole (US 6300353). It is obvious that this process using less expensive (S)- lactic acid makes the entire process with an inversion step even more complex than the original approach.
Elegant and more efficient process has been claimed in US 2004/0176432 from June 26, 2001 ) in which both chiral centers have been formed simultaneously, diastereo- and enantio-selectively pure in one single reaction step using chiral (R)-2-butynol as a chiral precursor in the presence of Pd(ll)-catalyst and diethyl zinc (Scheme 2).
Scheme 2:
Since water soluble, (R)-2-butynol is expensive, recently identical process has been published, in which instead of (R)-2-butynol less water soluble and therefore, less expensive (R)-4-phenyl-3-butyn-2-ol was used (Synthetic Commun. 39, 161 1 (2009)). Nevertheless, as incorrectly stated there, this process does not provide better diastereoselectivity than the original process using (R)-2-butynol: On the contrary disadvantage of this process is a very bad atom economy because huge phenyl group of (R)-4-phenyl-3-butyn-2-ol has to be “disposed” in oxidation step by the conversion of triple bond into carboxylic acid function.
……………………………
http://www.google.com/patents/WO2014023623A1?cl=en
The invention relates to a process for the manufacture of a
diastereomerically and enantiomerically enriched ester intermediate for isavuconazole or ravuconazole.
Isavuconazole and ravuconazole are triazole antifungal compounds. Processes for the manufacture of isavuconazole and ravuconazole were disclosed in patents WO99/45008, WO2007/062542 and WO03/002498 to Basilea. In WO2011/042827 a process for the manufacture of enantiomerically pure antifungal azoles such as ravuconazole and isavuconazole is disclosed, wherein a classical resolution of a racemic mixture is performed by the addition of an enantiopure chiral acid, then collection of the desired diastereomer followed by conversion of the salt into the enantiomerically pure form of the desired compound by treatment with a base or an ion-exchange resin. The disadvantages of using such classical resolution are that the chiral auxiliary needs to be applied in near stoichiometric amounts, and that additional process steps are required for recovery of these relatively high amounts of chiral reagent as well as for converting the salt into the free enantiopure product.
http://www.google.com/patents/US8076494
Reaction Scheme 1:
Filed under: Phase2 drugs Tagged: BMS-207147, ER-30346, phase 2, RAVUCONAZOLE








