Imatinib
Imatinib
CAS No:- [152459-95-5]
IUPAC Name:- 4-[(4-Methyl-1-piperazinyl)methyl]-N-[4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]phenyl]benzamide
M. P.:- 211-213 °C
MW: 493.604
4-[(4-methylpiperazin-1-yl)methyl]-N-(4-methyl-3-{[4-(pyridin-3-yl)pyrimidin-2-yl]amino}phenyl)benzamide
-[(4-methylpiperazin-1-yl)methyl]-N-(4-methyl-3-{[4-(pyridin-3-yl)pyrimidin-2-yl]amino}phenyl)benzamide
Imatinib (INN), marketed by Novartis as Gleevec (Canada, South Africa and the USA) or Glivec (Australia, Europe and Latin America), and sometimes referred to by its investigational name STI-571, is a tyrosine-kinase inhibitor used in the treatment of multiple cancers, most notably Philadelphia chromosome-positive (Ph+) chronic myelogenous leukemia (CML).[1]
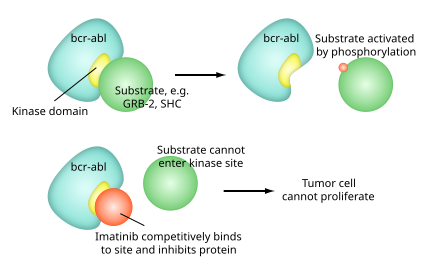
Like all tyrosine-kinase inhibitors, imatinib works by preventing a tyrosine kinase enzyme, in this case BCR-Abl, fromphosphorylating subsequent proteins and initiating the signalling cascade necessary for cancer growth and survival, thus preventing the growth of cancer cells and leading to their death by apoptosis.[2] Because the BCR-Abl tyrosine kinase enzyme exists only in cancer cells and not in healthy cells, imatinib works as a form of targeted therapy—only cancer cells are killed through the drug’s action.[3] In this regard, imatinib was one of the first cancer therapies to show the potential for such targeted action, and is often cited as a paradigm for research in cancer therapeutics.[4]
Imatinib has been cited as the first of the exceptionally expensive cancer drugs, costing $92,000 a year. Doctors and patients complain that this is excessive, given that its development costs have been recovered many times over, and that the costs of synthesizing the drug are orders of magnitude lower. In the USA, the patent protecting the active principle will expire on 4 January 2015 while the patent protecting the beta crystal form of the active principal ingredient will expire on 23 May 2019.[5]
The developers of imatinib were awarded the Lasker Award in 2009 and the Japan Prize in 2012.[6][7]

bcr-abl kinase (green), which causes CML, inhibited by imatinib (red; small molecule).
Medical uses
Imatinib is used to treat chronic myelogenous leukemia (CML), gastrointestinal stromal tumors (GISTs) and a number of othermalignancies.
Chronic myelogenous leukemia
The U.S. Food and Drug Administration (FDA) has approved imatinib as first-line treatment for Philadelphia chromosome-positive CML, both in adults and children. The drug is approved in multiple Philadelphia chromosome-positive cases of CML, including after stem cell transplant, in blast crisis, and newly diagnosed.[8]
Gastrointestinal stromal tumors
The FDA first granted approval for advanced GIST patients in 2002. On 1 February 2012, imatinib was approved for use after the surgical removal of KIT-positive tumors to help prevent recurrence.[9] The drug is also approved in unresectable KIT-positive GISTs.[8]
Other
The FDA has approved imatinib for use in adult patients with relapsed or refractory Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL), myelodysplastic/ myeloproliferative diseases associated with platelet-derived growth factor receptor gene rearrangements, aggressive systemic mastocytosis without or an unknown D816V c-KIT mutation, hypereosinophilic syndrome and/or chronic eosinophilic leukemia who have the FIP1L1-PDGFRα fusion kinase (CHIC2 allele deletion) or FIP1L1-PDGFRα fusion kinase negative or unknown, unresectable, recurrent and/or metastaticdermatofibrosarcoma protuberans.[8] On 25 January 2013, Gleevec was approved for use in children with Ph+ ALL.[10]
For treatment of progressive plexiform neurofibromas associated with neurofibromatosis type I, early research has shown potential for using the c-KIT tyrosine kinase blocking properties of imatinib.[11][12][13][14]
Legal challenge to generics
In 2007, imatinib became a test case through which Novartis challenged India’s patent laws. A win for Novartis would make it harder for Indian companies to produce generic versions of drugs still manufactured under patent elsewhere in the world. Doctors Without Borders argues a change in law would make it impossible for Indian companies to produce cheap generic antiretrovirals (anti-HIV medication), thus making it impossible for Third World countries to buy these essential medicines.[43] On 6 August 2007, the Madras High Court dismissed the writ petition filed by Novartis challenging the constitutionality of Section 3(d) of Indian Patent Act, and deferred to the World Trade Organization (WTO) forum to resolve the TRIPS compliance question. As of 2009 India has refused to grant patent exclusivity..
On April 01, 2013 Supreme Court of India dismissed the plea of Novartis for the grant of patent.

in germany
Mechanism of action
| Imatinib | |
|---|---|
| Drug mechanism | |

Crystallographic structure of tyrosine-protein kinase ABL (rainbow colored, N-terminus = blue, C-terminus = red) complexed with imatinib (spheres, carbon = white, oxygen = red, nitrogen = blue).[31]
|
|
| Therapeutic use | chronic myelogenous leukemia |
| Biological target | ABL, c-kit, PDGF-R |
| Mechanism of action | Tyrosine-kinase inhibitor |
| External links | |
| ATC code | L01XE01 |
| PDB ligand id | STI: PDBe, RCSB PDB |
| LIGPLOT | 1iep |
Imatinib is a 2-phenyl amino pyrimidine derivative that functions as a specific inhibitor of a number of tyrosine kinase enzymes. It occupies the TK active site, leading to a decrease in activity.
There are a large number of TK enzymes in the body, including the insulin receptor. Imatinib is specific for the TK domain inabl(the Abelson proto-oncogene), c-kit and PDGF-R (platelet-derived growth factorreceptor).
In chronic myelogenous leukemia, the Philadelphia chromosome leads to a fusion protein of abl with bcr(breakpoint cluster region), termed bcr-abl. As this is now aconstitutively active tyrosine kinase, imatinib is used to decrease bcr-abl activity.
The active sites of tyrosine kinases each have a binding site for ATP. The enzymatic activity catalyzed by a tyrosine kinase is the transfer of the terminal phosphate from ATP to tyrosine residues on its substrates, a process known as protein tyrosinephosphorylation. Imatinib works by binding close to the ATP binding site of bcr-abl, locking it in a closed or self-inhibited conformation, and therefore inhibiting the enzyme activity of the protein semi-competitively.[32] This fact explains why many BCR-ABL mutations can cause resistance to imatinib by shifting its equilibrium toward the open or active conformation.[33]
Imatinib is quite selective for bcr-abl – it does also inhibit other targets mentioned above (c-kit and PDGF-R), but no other knowntyrosine kinases. Imatinib also inhibits the abl protein of non-cancer cells but cells normally have additional redundant tyrosine kinases which allow them to continue to function even if abl tyrosine kinase is inhibited. Some tumor cells, however, have a dependence on bcr-abl.[34] Inhibition of the bcr-abl tyrosine kinase also stimulates its entry in to the nucleus, where it is unable to perform any of its normal anti-apoptopic functions.[35]
The Bcr-Abl pathway has many downstream pathways including the Ras/MapK pathway, which leads to increased proliferation due to increased growth factor-independent cell growth. It also affects the Src/Pax/Fak/Rac pathway. This affects the cytoskeleton, which leads to increased cell motility and decreased adhesion. The PI/PI3K/AKT/BCL-2 pathway is also affected. BCL-2 is responsible for keeping the mitochondria stable; this suppresses cell death by apoptosis and increases survival. The last pathway that Bcr-Abl affects is the JAK/STAT pathway, which is responsible for proliferation.[36]
synthesis
…………………………
Imatinib is known as an inhibitor of protein-tyrosine kinase and is indicated for the treatment of chronic myeloid leukemia (CML). Imatinib also has potential for the treatment of various other cancers that express these kinase including acute lymphocyte leukemia and certain solid tumors. It can also be used for the treatment of atherosclerosis, thrombosis, restenosis, or fibrosis. Thus, imatinib can also be used for the treatment of non-malignant diseases. Imatinib is usually administered orally in the form of a suitable salt, e.g., in the form of imatinib mesylate.
The chemical name of Imatinib is 4-(4-methyl piperazine -1- methyl) -N-4-methyl-3-[4- (3- pyridyl) pyrimidine-2-amino] – benzamide and is represented by the following structural formula:
(Imatinib)
Imatinib Mesylate is an inhibitor of signal transduction (STI571) invented by Novartis AG after 7 years of hard work; it is the first inhibitor of cancer signal transduction ratified in the whole world. It is sold by Novartis as Gleevec capsules containing imatinib mesylate in amounts equivalent to 100 mg or 400 mg of imatinib free base.
Imatinib Mesylate is the rare drug in America, European Union and Japan. In May 10, 2001, it was ratified by American Food and Drug Administration (FDA) to treat the chronic myelogenous leukemia patients. EP0564409 (US5521 184) describes the process for the preparation of imatinib and the use thereof, especially as an anti tumour agent.
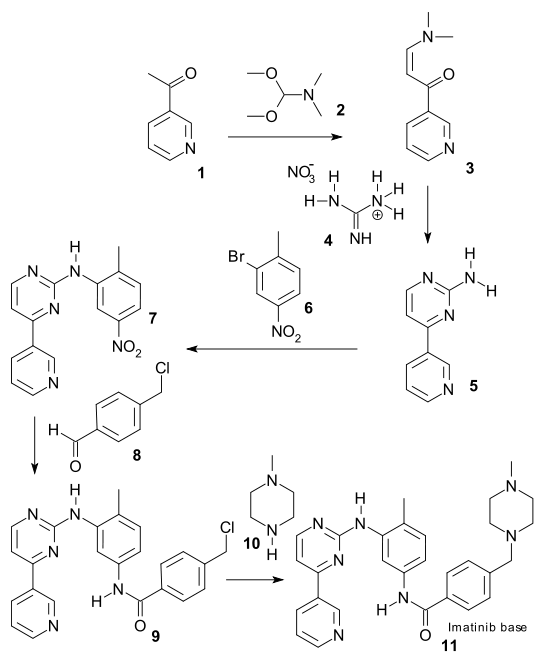
There are generally two synthetic routes for synthesis of Imatinib, suitable for the industrial production. One synthetic process as described in scheme-I comprises using 2-methyl-5-nitroaniline as the raw material which is reacted with cyanamide to obtain guanidine; cyclization reaction with 3-dimethylamino-l-(3-pyridyl)-2-propylene-l- ketone; reduction step of nitro to amine and condensation reaction with 4- (Chloromethyl)benzoyl chloride and N-methylpiperazidine to obtain Imatinib (WO 2004/108669). -I
Scheme-2 describes the successful process for the synthesis of Imatinib using 4-methyl-3- nitroanilines as the raw material, comprising reacting 4-methyl-3-nitroanilines with 4- (Chloromethyl)benzoyl chloride and N-methyl piperazidine in turns; followed by reduction of nitro group to amino group; then reaction with cyanamide to obtain guanidine; finally cyclization reaction with 3- dimethyl amino- 1 -(3- pyridyl)-2- propylene-1 -ketone to obtain Imatinib (WO 03/066613). The said PCT application discloses the use of 4-4-(methyl piperazin-l-ylmethyl)-benzoic acid methyl ester as one of the raw material but rest of the reactants are different from that of N-(5-amino -2- methylphenyl)-4-(3-pyridyl)-2-pyrimidine amine in presence of trimethyl aluminium.
Scheme-2
Common feature of the processes for preparing imatinib according to (WO 2004/108669) and (WO03/066613) lies in use of cyanamide as a reagent. The main difference between the two routes is that the reaction sequence of cyclization of pyrimidine chain is different. Example 10 of PCT International Publication no. WO 2003/066613 is less applicable to industrial purposes. These include the reaction between N-(3-bromo-4-methyl-phenyl)-4- (4-methyl-piperazin-l -ylmethyl)-benzamide and 4-(3-pyridyl)-2-pyrimidineamine which uses a mixture of rac-BINAP (a phosphine oxide catalyst) and Pd2 (dba)3*CHCl3. These catalysts are very expensive, therefore, their use is unfit for commercial production.
CN1630648A describes a process comprising reaction of 3- bromine-4- methyl aniline with 4-(4-methyl-piperazin- methyl) methyl benzoate in presence of trimethyl-Aluminum to obtain N-(4-methyl-3-bromobenzene)-4-(4-methyl-piperazin- 1 -methyl)-benzamide, which further reacts with 2-amino-4-(3-pyridyl)- pyrimidine in presence of palladium as catalyst to obtain Imatinib.
The drawback of the above process is the use of trimethyl-Aluminum, which is flammable and reacts severely when comes in contact with water.
CN101016293A describes another process using N-(4-methyl-3-3- aminophenyl)-4-(4- methyl-piperazin-1 -methyl)- benzamide as the raw material. The said raw material is reacted with 2-halogen-4-(3-pyridyl)- pyrimidine to obtain Imatinib.
The process disclosed in CN 101016293 A comprises use of halogenated agent, such as phosphorus oxychloride, which is used to synthesize 2-halogeno-4- methyl- (3-pyridyl) – pyridine is lachrymator and corrosive and has great influence to the surroundings. EP0564409 describes a coupling reaction between N-(5-amino -2-methylphenyl)-4-(3- pyridyl)-2-pyrimidine amine and 4-(4-methyl piperazin-l-ylmethyl)-benzoyl chloride in the presence of high quantity of pyridine to starting reactant amine N-(5-amino -2- methylphenyl)-4-(3-pyridyl)-2-pyrimidine amine. The ratio of the pyridine to the said reactant is 138 which is equivalent to about 40 parts v/w. Use of such a large quantity of pyridine is unsafe as it is a toxic solvent according to ICH guidelines. The workup of the reaction comprises evaporation of the remaining pyridine and further processing, which finally involves chromatography for purification, which is highly undesirable on industrial scale because it is expensive and time consuming.
US2006/0149061 and US20060223817 also discloses a similar synthetic approach comprising the use of similar pyridine /starting amine ratio (140 equivalents which is equals about 41 parts v/w). The product obtained is purified by slurring in ethyl acetate.
WO2004/074502 describes a coupling reaction between N-(5-amino -2-methylphenyl)-4- (3-pyridyl)-2-pyrimidine amine and 4-(4-methyl piperazin-l-ylmethyl)-benzoyl chloride wherein solvent like dimethyl pharmamide , dimethyl acetamide, N-methyl pyrilidinone are used as solvents instead of pyridine. However the method described in this patent application lacks an advantage in that the coupling reaction produces the hydrohalide salt of imatinib, e.g. imatinib trihydrochloride monohydrate, which has to be treated with a base in order to afford the imatinib base, thus an extra step is required. Further, conventional methods for coupling N-(5-amino -2-methylphenyl)-4-(3-pyridyl)-2- pyrimidine amine require reaction with an acid halide, e.g. 4-(4-methyl piperazin-1- ylmethyl)-benzoyl chloride, which requires an additional production step that can involve harsh and/or toxic chlorinating agent.
WO2008/1 17298 describes a coupling reaction between N-(5-amino -2-methylphenyl)-4- (3-pyridyl)-2-pyrimidine amine and 4-(4-methyl piperazin-l-ylmethyl)-benzoyl chloride in presence of a base selected from potassium carbonate, sodium carbonate, potassium or sodium hydroxide. Use of potassium carbonate as base results into the formation of Imatinib dihydrochloride which ultimately requires an additional operation of neutralization by using excessive base to get imatinib.
WO2008/136010 describes a coupling reaction between N-(5-amino -2-methylphenyl)-4- (3-pyridyl)-2-pyrimidine amine and 4-(4-methyl piperazin-l-ylmethyl)-benzoyl chloride in presence of base potassium hydroxide resulting into 78.6% yield of crude imatinib base. Preparation of crude requires imatinib hydrochloride preparation during the workup which is then basified to get base in crude form. This also describes maleate salt preparation as mode of purification which is again basified to give pure Imatinib base.
US patent application 2004/0248918 discloses a different approach using N-(5-amino -2- methylphenyl)-4-(3-pyridyl)-2-pyrimidine amine and 4-(2-chloromethyl)-benzoyl chloride as raw material. The reaction for the preparation of Imatinib is carried out in tetrahydrofuran as a reaction solvent and in the presence of pyridine as a base. However the method described in this patent application lacks an advantage as purification of the product requires column chromatography using chloroform: methanol (3: 1 v/v), which is not suitable purification method when performing the reaction on large scale, followed by crystallizati
US patent application 2008/0103305 discloses a process comprising reacting N-(5-amino -2-methylphenyl)-4-(3-pyridyl)-2-pyrimidine amine or its alkyl derivative and an acid salt of 4-[(4-methyl-l-piperazinyl)-methyl] benzoyl derivative as given below in the scheme-3 using pyridine in an amount of about 2 to 10 volumes per gram of the said amine. However the drawback associated with this process is use of pyridine especially when reaction is performed on large scale. -3
………………………….
SYNTHESIS
Inverse synthetic analysis will be divided into four imatinib into fragment A has 1,3 – parents electrical, fragment B are 1,3 – parent nuclear, fragments A and B constitute a pyrimidine ring.
Compound 4 can be obtained in two ways, benzyl bromide 1 and secondary amines 2 by SN2 reaction, or the aldehyde 3 with a secondary amine 2 by reductive amination. Sodium cyanoborohydride electron withdrawing effect of the cyano group, thereby reducing the activity of the negative hydrogen, it may be present in acidic solution. Also in the acidic conditions of aldehydes and secondary amines imine positive ions, which is higher than the activity of aldehyde reduction.This is why the reductive amination reagent with inert negative and hydrogen under acidic conditions. 4 hydrolyzed ester with thionyl chloride into the acid chloride 5 . The reaction of aniline and cyanamide dinucleophile guanidine 7 . Compound 8 and DMF-DMA reaction electrophilic reagent parents 9 , 7 , and 9 ring closure under alkaline conditions to generate 10 . Finally, reduction, amidation, and a salt of imatinib mesylate generated.
………………………………..

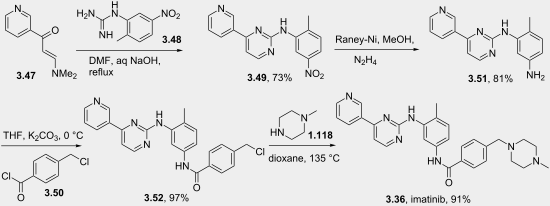
An efficient, economic process has been developed for the production of imatinib with 99.99% purity and 50% overall yield from four steps. Formation and control of all possible impurities is described. The synthesis comprises the condensation of N-(5-amino-2-methylphenyl)-4-(3-pyridinyl)-2-pyrimidineamine with 4-(4-methylpiperazinomethyl)benzoyl chloride in isopropyl alcohol solvent in the presence of potassium carbonate to yield imatinib base.
…………………………
DOI: 10.1039/C2OB27003J
http://pubs.rsc.org/en/content/articlelanding/2013/ob/c2ob27003j#!divAbstract
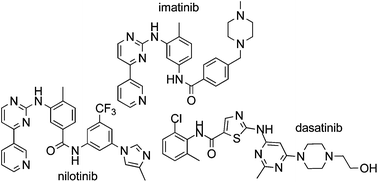
Imatinib (1), nilotinib (2) and dasatinib (3) are Bcr-Abl tyrosine kinase inhibitors approved for the treatment of chronic myelogenous leukemia (CML). This review collates information from the journal and patent literature to provide a comprehensive reference source of the different synthetic methods used to prepare the aforementioned active pharmaceutical ingredients (API’s).
……………………..
|
Medicine for Blood Cancer
‘Imitinef Mercilet’ is a medicine which cures blood cancer.
Its available free of cost at “Adyar Cancer Institute in Chennai”.
Create Awareness. It might help someone.Cancer Institute in Adyar, Chennai
‘Imitinef Mercilet’ is apparently an alternative spelling of the drug Imatinib mesylate which is used in the treatment of some forms of leukemia along with other types of cancer. Imatinib, often referred to a “Gleevec”, has proved to be an effective treatment for some forms of cancers. However, “blood cancer” is a generalized term for cancers that affect the blood, lymphatic system or bone marrow. The three types of blood cancer are listed as leukemia, lymphoma, and multiple myeloma. These three malignancies require quite different kinds of treatments. While drugs (including Imatinib), along with other treatments such as radiation can help to slow or even stop the progress of these cancers, there is currently no single drug treatment that can be said to actually cure all such cancers.
Category: Cancer
Address: East Canal Bank Road , Gandhi Nagar
Adyar, Chennai -600020
Landmark: Near Michael School
Phone: 044-24910754 044-24910754 ,
044-24911526 044-24911526 , 044-22350241
Imatinib is a small molecule selectively inhibiting specific tyrosine kinases that has emerged recently as a valuable treatment for patients with advanced GIST. The use of imatinib as monotherapy for the treatment of GIST has been described in PCT publication WO 02/34727, which is here incorporated by reference. However, it has been reported that primary resistance to imatinib is present in a population of patients, for example 13.7% of patients in one study. In addition, a number of patients acquire resistance to treatment with imatinib. More generally this resistance is partial with progression in some lesions, but continuing disease control in other lesions. Hence, these patients remain on imatinib treatment but with a clear need for additional or alternative therapy.
Imatinib is 4-(4-methylpiperazin-1-ylmethyl)-N-[4-methyl-3-(4-pyridin-3-yl)pyrimidin-2-ylamino)phenyl]-benzamide having the formula I
The preparation of imatinib and the use thereof, especially as an anti-tumour agent, are described in Example 21 of European patent application EP-A-0 564 409, which was published on 6 Oct. 1993, and in equivalent applications and patents in numerous other countries, e.g. in U.S. Pat. No. 5,521,184 and in Japanese patent 2706682

The flow-based route required minimal manual intervention and was achieved despite poor solubility of many reaction components

UK chemists have used a combination of flow chemistry methods with solid-supported scavengers and reagents to synthesise the active pharmaceutical ingredient, imatinib, of the anticancer drug Gleevec. The method avoids the need for any manual handling of intermediates and allows the drug to be synthesised in high purity in less than a day.
Gleevec, developed by Novartis, is a tyrosine kinase inhibitor used for the treatment of chronic myeloid leukaemia and gastrointestinal stromal tumours.

READ ALL AT
http://www.rsc.org/chemistryworld/2013/01/flow-synthesis-anticancer-drug
 |
|
|---|---|
 |
|
| IMATINIB |
CREDIT
http://www.veomed.com/va041542042010

‘Wrapping’ Gleevec Fights Drug-Resistant Cancer, Study Shows
http://www.sciencedaily.com/releases/2007/05/070501115127.htm
The anti-cancer drug Gleevec® is far more effective against a drug-resistant strain of cancer when the drug wraps the target with a molecular bandage that seals out water from a critical area. This image shows the bandage (black box) on the modified version of the drug, WBZ-7. (Credit: Image courtesy of Rice University)
A new study in Cancer Research finds that the anti-cancer drug Gleevec® is far more effective against a drug-resistant strain of cancer when the drug wraps the target with a molecular bandage that seals out water from a critical area.

FIG 23.8 Optimization of imatinib as a chemotherapeutic agent. The discovery that 2-phenylaminopyrimidine inhibitors of PKC also inhibit the unrelated v-Abl oncogene turned attention to its potential use in the treatment of chronic myelogenous leukaemia. Starting with the 2-phenylaminopyrimidine backbone, addition of the benzamidine group increased activity against tyrosine kinases, the methyl group reduced its activity against PKC (so-called ‘ target hopping ’ ). Addition of a 3’-pyridyl group improved the activity in cellular assays. Subsequent addition of N -methylpiperazine increased water solubility and oral bioavailability, enabling the drug to survive the stomach and to enter the bloodstream.
……………………..
An automated flow-based synthesis of imatinib: the API of gleevec M.D. Hopkin, I.R. Baxendale, S.V. Ley, J.C.S. Chem. Commun.2010, 46, 2450-2452.

References
- Jump up^ Novartis Pharma AG. Gleevec® (imatinib mesylate) tablets prescribing information. East Hanover, NJ; 2006 Sep. Anon. Drugs of choice for cancer. Treat Guidel Med Lett. 2003; 1:41–52
- Jump up^ Goldman JM, Melo JV (October 2003). “Chronic myeloid leukemia–advances in biology and new approaches to treatment”. N. Engl. J. Med. 349 (15): 1451–64.doi:10.1056/NEJMra020777. PMID 14534339.
- Jump up^ Fausel, C. Targeted chronic myeloid leukemia therapy: Seeking a cure. Am J Health Syst Pharm 64, S9-15 (2007)
- Jump up^ Stegmeier F, Warmuth M, Sellers WR, Dorsch M (May 2010). “Targeted cancer therapies in the twenty-first century: lessons from imatinib”. Clin. Pharmacol. Ther. 87(5): 543–52. doi:10.1038/clpt.2009.297. PMID 20237469.
- Jump up^ “Novartis fails to patent Glivec (Gleevec) in India”.
- Jump up^ Rowley to receive Japan Prize for her role in the development of targeted cancer therapy Eurekalert, Press release, 24 January 2012
- Jump up^ Leukemia Drug and Magnet Material Net Japan Prizes by Dennis Normile, Science Insider, 25 January 2012
- ^ Jump up to:a b c “FDA Highlights and Prescribing Information for Gleevec(imatinib mesylate)”.
- Jump up^ “Prolonged Use of Imatinib in GIST Patients Leads to New FDA Approval”.
- Jump up^ “FDA approves Gleevec for children with acute lymphoblastic leukemia”. FDA News Release. US Food and Drug Administration. 25 January 2013. Retrieved 3 April 2013.
- Jump up^ Yang FC, Ingram DA, Chen S, Zhu Y, Yuan J, Li X, Yang X, Knowles S, Horn W, Li Y, Zhang S, Yang Y, Vakili ST, Yu M, Burns D, Robertson K, Hutchins G, Parada LF, Clapp DW (October 2008). “Nf1-dependent tumors require a microenvironment containing Nf1+/–and c-kit-dependent bone marrow”. Cell 135 (3): 437–48.doi:10.1016/j.cell.2008.08.041. PMC 2788814. PMID 18984156. Lay summary –Science Daily.
- Jump up^ “Gleevec NF1 Trial”. Nfcure.org. Retrieved 2013-04-03.
- Jump up^ “GIST in Neurofibromatosis 1″. Gistsupport.org. 2010-05-14. Retrieved 2013-04-03.
- Jump up^ “”Pilot Study of Gleevec/Imatinib Mesylate (STI-571, NSC 716051) in Neurofibromatosis (NF1) Patient With Plexiform Neurofibromas (0908-09)” (Suspended)”. Clinicaltrials.gov. Retrieved 2013-04-03.
- Jump up^ Droogendijk HJ, Kluin-Nelemans HJ, van Doormaal JJ, Oranje AP, van de Loosdrecht AA, van Daele PL (July 2006). “Imatinib mesylate in the treatment of systemic mastocytosis: a phase II trial”. Cancer 107 (2): 345–51. doi:10.1002/cncr.21996.PMID 16779792.
- Jump up^ Tapper EB, Knowles D, Heffron T, Lawrence EC, Csete M (June 2009). “Portopulmonary hypertension: imatinib as a novel treatment and the Emory experience with this condition”. Transplant. Proc. 41 (5): 1969–71.doi:10.1016/j.transproceed.2009.02.100. PMID 19545770.
- Jump up^ Boucher P, Gotthardt M, Li WP, Anderson RG, Herz J (April 2003). “LRP: role in vascular wall integrity and protection from atherosclerosis”. Science 300 (5617): 329–32.doi:10.1126/science.1082095. PMID 12690199.
- Jump up^ Lassila M, Allen TJ, Cao Z, Thallas V, Jandeleit-Dahm KA, Candido R, Cooper ME (May 2004). “Imatinib attenuates diabetes-associated atherosclerosis”. Arterioscler. Thromb. Vasc. Biol. 24 (5): 935–42. doi:10.1161/01.ATV.0000124105.39900.db.PMID 14988091.
- Jump up^ Reeves PM, Bommarius B, Lebeis S, McNulty S, Christensen J, Swimm A, Chahroudi A, Chavan R, Feinberg MB, Veach D, Bornmann W, Sherman M, Kalman D (July 2005). “Disabling poxvirus pathogenesis by inhibition of Abl-family tyrosine kinases”. Nat. Med.11 (7): 731–9. doi:10.1038/nm1265. PMID 15980865.
- Jump up^ He G, Luo W, Li P, Remmers C, Netzer WJ, Hendrick J, Bettayeb K, Flajolet M, Gorelick F, Wennogle LP, Greengard P (September 2010). “Gamma-secretase activating protein is a therapeutic target for Alzheimer’s disease”. Nature 467 (7311): 95–8.doi:10.1038/nature09325. PMC 2936959. PMID 20811458.
- Jump up^ “Alzheimer’s may start in liver – Health – Alzheimer’s Disease | NBC News”. MSNBC. Retrieved 2013-01-06.
- Jump up^ Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, Jones RW, Bullock R, Love S, Neal JW, Zotova E, Nicoll JA (July 2008). “Long-term effects of Abeta42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial”. Lancet 372 (9634): 216–23. doi:10.1016/S0140-6736(08)61075-2. PMID 18640458.
- Jump up^ Eliminating Morphine Tolerance – Reformulated Imatinib 23 Feb 2012, 5:00 PST
- Jump up^ “GLIVEC Tablets – Summary of Product Characteristics (SPC)”. electronic Medicines Compendium. Novartis Pharmaceuticals UK Ltd.
- ^ Jump up to:a b c “Gleevec (imatinib) dosing, indications, interactions, adverse effects, and more”.Medscape Reference. WebMD. Retrieved 24 January 2014.
- Jump up^ “Imatinib”. Macmillan Cancer Support. Retrieved 26 December 2012.
- ^ Jump up to:a b Haberfeld, H, ed. (2009). Austria-Codex (in German) (2009/2010 ed.). Vienna: Österreichischer Apothekerverlag. ISBN 3-85200-196-X.
- Jump up^ Kerkelä R, Grazette L, Yacobi R, Iliescu C, Patten R, Beahm C, Walters B, Shevtsov S, Pesant S, Clubb FJ, Rosenzweig A, Salomon RN, Van Etten RA, Alroy J, Durand JB, Force T (August 2006). “Cardiotoxicity of the cancer therapeutic agent imatinib mesylate”. Nat. Med. 12 (8): 908–16. doi:10.1038/nm1446. PMID 16862153.
- Jump up^ Shima H, Tokuyama M, Tanizawa A, Tono C, Hamamoto K, Muramatsu H, Watanabe A, Hotta N, Ito M, Kurosawa H, Kato K, Tsurusawa M, Horibe K, Shimada H (October 2011). “Distinct impact of imatinib on growth at prepubertal and pubertal ages of children with chronic myeloid leukemia”. J. Pediatr. 159 (4): 676–81.doi:10.1016/j.jpeds.2011.03.046. PMID 21592517.
- ^ Jump up to:a b c d “GLIVEC (imatinib)” (PDF). TGA eBusiness Services. Novartis Pharmaceuticals Australia Pty Ltd. 21 August 2013. Retrieved 24 January 2014.
- Jump up^ PDB 1IEP; Nagar B, Bornmann WG, Pellicena P, Schindler T, Veach DR, Miller WT, Clarkson B, Kuriyan J (August 2002). “Crystal structures of the kinase domain of c-Abl in complex with the small molecule inhibitors PD173955 and imatinib (STI-571)”. Cancer Res. 62 (15): 4236–43. PMID 12154025.
- Jump up^ Takimoto CH, Calvo E. “Principles of Oncologic Pharmacotherapy” in Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ (Eds)Cancer Management: A Multidisciplinary Approach. 11 ed. 2008.
- Jump up^ Gambacorti-Passerini CB, Gunby RH, Piazza R, Galietta A, Rostagno R, Scapozza L (February 2003). “Molecular mechanisms of resistance to imatinib in Philadelphia-chromosome-positive leukaemias”. Lancet Oncol. 4 (2): 75–85. doi:10.1016/S1470-2045(03)00979-3. PMID 12573349.
- Jump up^ Deininger MW, Druker BJ (September 2003). “Specific targeted therapy of chronic myelogenous leukemia with imatinib”. Pharmacol. Rev. 55 (3): 401–23.doi:10.1124/pr.55.3.4. PMID 12869662.
- Jump up^ Vigneri P, Wang JY (February 2001). “Induction of apoptosis in chronic myelogenous leukemia cells through nuclear entrapment of BCR-ABL tyrosine kinase”. Nat. Med. 7 (2): 228–34. doi:10.1038/84683. PMID 11175855.
- Jump up^ Weisberg E, Manley PW, Cowan-Jacob SW, Hochhaus A, Griffin JD (May 2007). “Second generation inhibitors of BCR-ABL for the treatment of imatinib-resistant chronic myeloid leukaemia”. Nature Reviews Cancer 7 (5): 345–56. doi:10.1038/nrc2126.PMID 17457302.
- Jump up^ Scheinfeld N, Schienfeld N (February 2006). “A comprehensive review of imatinib mesylate (Gleevec) for dermatological diseases”. J Drugs Dermatol 5 (2): 117–22.PMID 16485879.
- Jump up^ Klopp, T, ed. (2010). Arzneimittel-Interaktionen (in German) (2010/2011 ed.). Arbeitsgemeinschaft für Pharmazeutische Information. ISBN 978-3-85200-207-1.
- ^ Jump up to:a b Staff, Innovation.org (a project of the Pharmaceutical Research and Manufacturers of America)The Story of Gleevec
- Jump up^ Claudia Dreifus for the New York Times. November 2, 2009 Researcher Behind the Drug Gleevec
- ^ Jump up to:a b A Conversation With Brian J. Druker, M.D., Researcher Behind the Drug Gleevecby Claudia Dreifus, The New York Times, 2 November 2009
- Jump up^ Gambacorti-Passerini C (2008). “Part I: Milestones in personalised medicine—imatinib”. Lancet Oncology 9 (600): 600. doi:10.1016/S1470-2045(08)70152-9.PMID 18510992.
- Jump up^ Druker BJ, Lydon NB (January 2000). “Lessons learned from the development of an abl tyrosine kinase inhibitor for chronic myelogenous leukemia”. J. Clin. Invest. 105 (1): 3–7. doi:10.1172/JCI9083. PMC 382593. PMID 10619854.
- ^ Jump up to:a b c U.S. Patent 5,521,184
- Jump up^ “Imatinib Patent Family”. Espacenet. 1996. Retrieved 2014-07-23.
- ^ Jump up to:a b EP 0564409
- Jump up^ Staff, European Medicines Agency, 2004.EMEA Scientific Discussion of Glivec
- Jump up^ Note: The Indian patent application, which became the subject of litigation in India that gathered a lot of press, does not appear to be publicly available. However according todocuments produced in the course of that litigation (page 27), “The Appellant’s application under the PCT was substantially on the same invention as had been made in India.”
- ^ Jump up to:a b WO 9903854
- Jump up^ U.S. Patent 6,894,051
- Jump up^ FDA Orange Book; Patent and Exclusivity Search Results from query on Appl No 021588 Product 001 in the OB_Rx list.
- Jump up^ Novartis press release, May 10, 2001. [http://www.evaluategroup.com/Universal/View.aspx?type=Story&id=5838 FDA approves Novartis’ unique cancer medication Glivec®
- Jump up^ Cohen MH et al. Approval Summary for Imatinib Mesylate Capsules in the Treatment of Chronic Myelogenous Leukemia Clin Cancer Res May 2002 8; 935
- Jump up^ Margot J. Fromer for Oncology Times. December 2002. What’s in a Name? Quite a Lot When It Comes to Marketing & Selling New Cancer Drugs
- Jump up^ Novartis Press Release. April 30 2001Novartis Oncology Changes Trade Name of Investigational Agent Glivec(TM) to Gleevec(TM) in the United States
- Jump up^ Experts in Chronic Myeloid Leukemia. The price of drugs for chronic myeloid leukemia (CML) is a reflection of the unsustainable prices of cancer drugs: from the perspective of a large group of CML experts Blood. 2013 May 30;121(22):4439-42. PMID 23620577
- Jump up^ Andrew Pollack for the New York Times, April 25, 2013 Doctors Denounce Cancer Drug Prices of $100,000 a Year
- Jump up^ Schiffer CA (July 2007). “BCR-ABL tyrosine kinase inhibitors for chronic myelogenous leukemia”. N. Engl. J. Med. 357 (3): 258–65. doi:10.1056/NEJMct071828.PMID 17634461.
- Jump up^ As Pills Treat Cancer, Insurance Lags Behind, By ANDREW POLLACK, New York Times, 14 April 2009
- Jump up^ Living With a Formerly fatal Blood Cancer, By JANE E. BRODY, New York Times, 18 January 2010
- Jump up^ Patented Medicine Review Board (Canada). Report on New Patented Drugs – Gleevec.
- Jump up^ “pharmacychecker.com”. pharmacychecker.com. Retrieved 2013-04-03.
- Jump up^ Gardiner Harris and Katie Thomas for the New York Times. April 1 2013 Top court in India rejects Novartis drug patent
- Jump up^ Note: The Indian patent application No.1602/MAS/1998 does not appear to be publicly available. However according to the decision of the IPAB on 26 June 2009 (page 27) discussed below, “The Appellant’s application under the PCT was substantially on the same invention as had been made in India.”
- Jump up^ Staff, European Medicines Agency, 2004. EMEA Scientific Discussion of Glivec
- Jump up^ Indian Supreme Court Decision paragraphs 5-6
- Jump up^ Novartis v UoI, para 8-9
- ^ Jump up to:a b Shamnad Basheer for Spicy IP March 11, 2006First Mailbox Opposition (Gleevec) Decided in India
- Jump up^ Staff, LawyersCollective. September 6, 2011[http://www.lawyerscollective.org/news/archived-news-a-articles/126-novartis-case-background-and-update-supreme-court-of-india-to-recommence-hearing.html Novartis case: background and update – Supreme Court of India to recommence hearing
- Jump up^ R. Jai Krishna and Jeanne Whalen for the Wall Street Journal. April 1, 2013Novartis Loses Glivec Patent Battle in India
- Jump up^ Intellectual Property Appellate Board decision dated 26 June 2009, p 149
- Jump up^ W.P. No.24759 of 2006
- Jump up^ “Supreme Court rejects bid by Novartis to patent Glivec”.
- Jump up^ Novartis v UoI, Para 191
- Jump up^ Novartis v UoI, Para 24-25
- Jump up^ “How the Indian judgment will reverberate across the world”.
- Jump up^ “Patented drugs must be priced smartly”.
- Jump up^ Patent with a purpose, Prof. Shamnad Basheer, Indian Express, 3 April 2013
- Jump up^ Kevin Grogan for PharmaTimes. February 27, 2012 Novartis explains stance over India patent law challenge
- Jump up^ Berne Declaration. May 8, 2007 Short questions and answers about the court case initiated by Novartis in India
External links
Filed under: cancer Tagged: IMATINIB



















![[1860-5397-9-265-i38]](http://www.beilstein-journals.org/bjoc/content/inline/1860-5397-9-265-i38.png?max-width=550&background=EEEEEE)