
Ridgefield, Conn., September 25, 2014 – Boehringer Ingelheim Pharmaceuticals, Inc. announced today that the U.S. Food and Drug Administration (FDA) approved Spiriva Respimat (tiotropium bromide) inhalation spray for the long-term, once-daily maintenance treatment of bronchospasm associated with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and emphysema and to reduce exacerbations in COPD patients. Boehringer Ingelheim anticipates Spiriva Respimat to be available in January 2015.
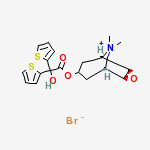
Spiriva Respimat provides a pre-measured amount of medicine in a slow-moving mist that helps patients inhale the medicine. Spiriva Respimat was developed to actively deliver medication in a way that does not depend of how fast air is breathed in from the inhaler.
READ AT


MAKE IN INDIA
http://makeinindia.com/sector/pharmaceuticals/
Filed under: FDA 2014 Tagged: Boehringer Ingelheim, Boehringer Ingelheim Pharmaceuticals., Chronic Obstructive Pulmonary Disease, COPD, FDA 2014, Food and Drug Administration, Inc., Spiriva, Spiriva Respimat, tiotropium, tiotropium bromide

