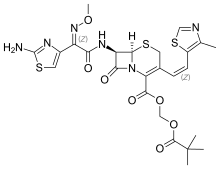
Cefditoren pivoxil
ME-1207, Spectracef, Meiact
117467-28-4
- (-)-(6R,7R)-2,2-dimethylpropionyloxymethyl 7-((Z)-2-(2-aminothiazol-4-yl)-2-methoxyiminoacetamido)-3-((Z)-2-(4-methylthiazol-5-yl)ethenyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylate
- 7-(2-(2-aminothiazol-4-yl)-2-(methoxyimino)acetamido)-3-(2-(4-methylthiazol-5-yl)ethenyl)cephem-4-carboxylic acid pivaloyloxymethyl ester
- CDTR-PI
- cefditoren pivoxil
- ME 1207
- ME-1207
- Spectracef
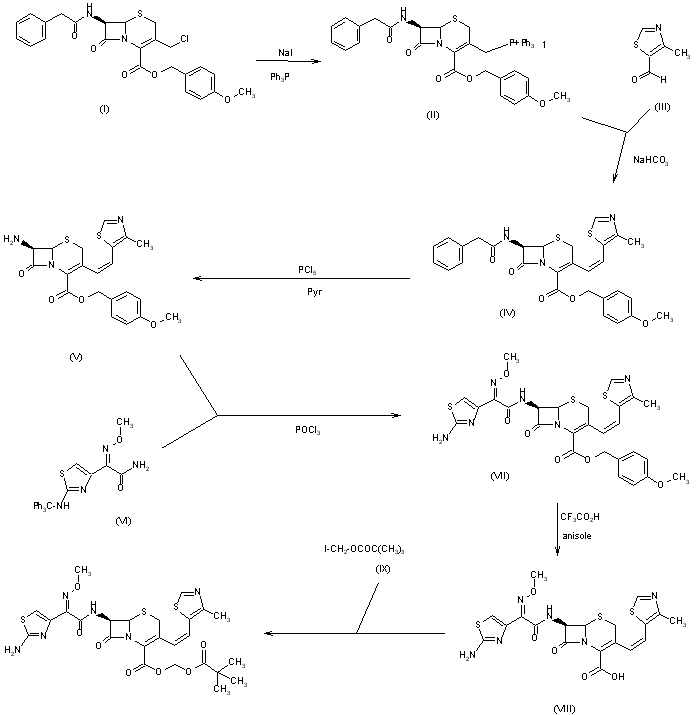
Novel crystalline form of cefditoren pivoxil (first disclosed in EP175610). Represents Yungjin Pharma’s first interest in this API, which was developed and launched by Meiji Seika and previous licensee TAP Pharmaceuticals, and now marketed by Merus Labs, for treating chronic bronchitis and community acquired pneumonia caused by bacterial infections
Cefditoren is a third-generation cephalosporin antibiotic for oral use. It is commonly marketed under the trade name Spectracef by Vansen Pharma Inc.
Cefditoren is also marketed under the name Meiact by Meiji Seika Pharma Co., Ltd.[1]
Spectrum of bacterial susceptibility
Cefditoren has a broad spectrum of activity and has been used to treat bacterial infections of the skin and respiratory tract, including bronchitis, pneumonia, and tonsillitis. The following represents MIC susceptibility data for a few medically significant microorganisms.
- Haemophilus influenzae: ≥0.063 – 0.25 μg/ml
- Staphylcoccus aureus: 0.25 – >128 μg/ml (includes MRSA)
- Streptococcus pyogenes: ≤0.004 – 2 μg/ml[2]
Cefditoren is a broad-spectrum antibiotic against Gram-negative and Gram-positive bacteria, but does not have antibacterial activity against Pseudomonas aeruginosa.[3]
Clinical use
Indications
Cefditoren is used to treat uncomplicated skin and skin structure infections, community-acquired pneumonia, acute bacterial exacerbation of chronic bronchitis, pharyngitis, and tonsillitis.
Formulations
Cefditoren is available as 200- and 400-mg tablets. It can be formulated as the prodrug cefditoren pivoxil.
References
- Meiact Full Description
- http://www.toku-e.com/Assets/MIC/Cefditoren%20sodium.pdf
- “Disease relevance of Cefditoren”. Retrieved June 24, 2014.
- Chem Pharm Bull 1992,39(9),2433
- J Antibiot 1990,43(8),1047
Synthesis Reference
Kiyoshi Yasui, Masahiro Onodera, Masamichi Sukegawa, Tatsuo Watanabe, Yuichi Yamamoto, Yasushi Murai, Katsuharu Iinuma, “Crystalline substance of cefditoren pivoxyl and the production of the same.” U.S. Patent US6294669, issued March, 1986.
| Patent | Submitted | Granted |
|---|---|---|
| Therapy for Treating Resistant Bacterial Infections [US2009275552] | 2009-11-05 | |
| Process for the preparation of thiazole intermediate [US6833459] | 2003-10-30 | 2004-12-21 |
| Nanoparticulate and Controlled Release Compositions Comprising Cefditoren [US8119163] | 2008-11-13 | 2012-02-21 |
External links
 |
|
| Systematic (IUPAC) name | |
|---|---|
| (7R)-7-((Z)-2-(2-Aminothiazol-4-yl)-2-(methoxyimino)acetamido)-3-((Z)-2-(4-methylthiazol-5-yl)vinyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid | |
| Clinical data | |
| Trade names | Spectracef |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a605003 |
| Legal status |
?
|
| Identifiers | |
| CAS number | 104145-95-1  |
| ATC code | J01DD16 |
| PubChem | CID 9870843 |
| DrugBank | DB01066 |
| ChemSpider | 8046534  |
| UNII | 81QS09V3YW  |
| Chemical data | |
| Formula | C19H18N6O5S3 |
| Mol. mass | 506.58 g/mol |
WO-2014189308, Yungjin Pharmaceutical Co Ltd
Filed under: Uncategorized Tagged: Cefditoren pivoxil