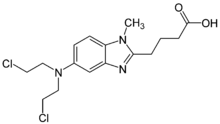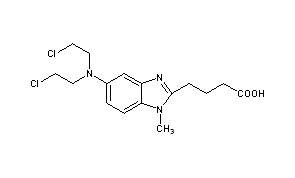

TREANDA contains bendamustine hydrochloride, an alkylating drug, as the active ingredient. The chemical name of bendamustine hydrochloride is 1H-benzimidazole-2-butanoic acid, 5-[bis(2-chloroethyl)amino]-1 methyl-, monohydrochloride. Its empirical molecular formula is C16H21Cl2N3O2 • HCl, and the molecular weight is 394.7. Bendamustine hydrochloride contains a mechlorethamine group and a benzimidazole heterocyclic ring with a butyric acid substituent, and has the following structural formula:
 |
TREANDA (bendamustine hydrochloride) for Injection is intended for intravenous infusion only after reconstitution with Sterile Water for Injection, USP, and after further dilution with either 0.9% Sodium Chloride Injection, USP, or 2.5% Dextrose/0.45% Sodium Chloride Injection, USP. It is supplied as a sterile non-pyrogenic white to off-white lyophilized powder in a single-use vial. Each 25-mg vial contains 25 mg of bendamustine hydrochloride and 42.5 mg of mannitol, USP. Each 100-mg vial contains 100 mg of bendamustine hydrochloride and 170 mg of mannitol, USP. The pH of the reconstituted solution is 2.5 -3.5.
Bendamustine hydrochloride, 4-{5-[Bis(2-chloroethyl) amino]- l-methyl-2- benzimidazolyl} butyric acid hydrochloride, of the formula (VI) :
was initially synthesized in 1963 in the German Democratic Republic (GDR) and was available from 1971 to 1992 there, as the hydrochloride salt, under the trade name Cytostasan®. Since that time, it has been marketed in Germany under the trade name Ribomustin®. Bendamustine Hydrochloride as injection is available in the United States under the tradename Treanda®. Bendamustine hydrochloride is an alkylating agent that is approved for the treatment of non-Hodgkin’s lymphoma, multiple myeloma and chronic lymphocytic leukemia.
Bendamustine hydrochloride is a benzimidazole analog. While bendamustine has been demonstrated as efficacious, it is known to be unstable, especially in aqueous solutions, leading to formation of non-bendamustine products (i.e. “degradation impurities”) which leads to technical difficulties in its preparation and administration. In light of its instability in aqueous solution, bendamustine is supplied as a lyophilized cake of bendamustine hydrochloride salt. US2006/159713, US 2006/128777 and WO2010/036702 disclose various impurities of Bendamustine hydrochloride which are as follows:
PC-1 PC-2
Jena et al. were the first to disclose the synthesis of Bendamustine hydrochloride in German (GDR) Patent No. 34727. Krueger et al. in German (GDR) Patent No. 159877 recite a method as summarized in scheme-1, for the synthesis of bendamustine hydrochloride comprising the reaction of the 4-[l-methyl-5-bis-(2- hydroxyethyl)-benzimidazolyl-2]butyric acid ethyl ester (4) (or the corresponding methyl, propyl or butyl ester) with thionyl chloride in chloroform at 0-5°C to form 4-[l- methyl-5-bis-(2-chloroethyl)-benzimidazolyl-2]butyric acid ethyl ester (5). Excess of thionyl chloride is destroyed by stirring the reaction mixture in aqueous HCl. Finally chloroform is distilled off and stirred at 95°C for 3 hours. The reaction mixture is partially concentrated and the residue is diluted with water and stirred upto crystallization. Further purification is done by recrystallization from water.
Scheme-1: Method disclosed by Krueger et al. in DD159877 for the synthesis of Bendamustine hydrochloride
Bendamustine hydrochloride (6)
Ozegowski et al in Zentralblatt fuer Pharmazie, Pharmakotherapie und Laboratoriumsdiagnostik 1 10 (10), 1013-1019 (1971) discloses a process for the preparation of bendamustine hydrochloride monohydrate. The Chinese journal “Chinese journal of New Drugs “, 2007, No. 23, Vol. 16, 1960-61 and J. Prakt. Chem. 20, 178-186 (1963) disclose another method for the synthesis of Bendamustine hydrochloride monohydrate starting from 2,4-dinitrochlorobenzene as summarized in scheme-2.
The crucial conversions are reaction of l-methyl-2-(4′-ethyl butyrate)-5- amino]-lH-benzimidazole 6 with ethylene oxide in the presence of water, sodium acetate and acetic acid, by maintaining at 5°C for 5 hours and overnight at 20°C to give 4-{5-[bis-(2-hydroxy-ethyl)-amino]-l-methyl-lH-benzimidazol-2-yl}-butyric acid ethyl ester (dihydroxy ester) 7 as a jelly mass, which on chlorination using thionyl chloride in chloroform and subsequent in situ hydrolysis with concentrated HCI gave bendamustine hydrochloride. It also discloses a process for the recrystallization of bendamustine hydrochloride from water and the product obtained is a monohydrate with a melting point of 148-151°C.
IP.com Journal 2009, 9(7B), 21 discloses another process as shown below for the preparation of ethyl-4-[5-[bis(2-hydroxyethyl) amino]- l-methylbenzimidazol-2- yl]butanoate (III) wherein ethyl-4-(5 -amino- 1 -methyl- lH-benzo[d]imidazol-2-yl) butanoate (II) is reacted with 2-halo ethanol in the presence of an inorganic base selected from the group consisting potassium carbonate, potassium bicarbonate, sodium
The PCT application WO 2010/042568 assigned to Cephalon discloses the synthesis of Bendamustine hydrochloride as summarized in schem-3 starting from 2,4- dintroaniline in six steps. The crucial step is reductive alkylation of Il-a, using borane- tetrahydrofuran and chloroacetic acid at ambient temperature, producing compound of formula I-a. Acid mediated hydrolysis of I-a using concentrated hydrochloric acid at reflux produced bendamustine hydrochloride which has a purity of 99.1%. The above PCT Patent application also discloses a method of purification of Bendamustine hydrochloride by agitating the Bendamustine hydrochloride in a mixture of DMF and THF at 75°C for about 30 minutes followed by cooling to ambient temperature and isolating the solid by filtration.
Scheme-3:
iil-a
Bemdamuatine hydrochloride
The PCT application WO 2011/079193 assigned to Dr. Reddy’s Laboratories discloses the synthesis of Bendamustine hydrochloride as summarized in schem-4 starting from compound of formula (II). The crucial step is alkylation of compound of formula II with 2-haloethanol in the presence of an organic base to give a compound of formula (III) which on chlorination with a chlorinating agent affords a compound of formula (IV). Compound of formula (IV) on hydrolysis in acidic medium gives bendamustine hydrochloride. It further discloses purification of bendamustine hydrochloride using aqueous hydrochloric acid and acetonitrile.
Scheme-4:
Bendamustine hydrochloride (Pure)
The most of the prior art processes described above involve
• The use of ethylene oxide for the preparation of bendamustine hydrochloride, which is often not suitable for industrial scale processes due to difficulty in handling ethylene oxide, since it is shipped as a refrigerated liquid.
• Further, the known processes involve the use of strongly acidic conditions and high temperatures for the hydrolysis of ethyl ester of bendamustine and subsequent in-situ formation of bendamustine hydrochloride, thereby resulting in increased levels of various process-related impurities IMP. -A (RRT-0.46), IMP. -B (RRT-1.27) and IMP. -C (RRT-1.31) whose removal is quite difficult and make the process less economically viable.
IMP.-B
International Application Publication No. WO 2009/120386 describes various solid forms of bendamustine hydrochloride designated as bendamustine hydrochloride Form 1, bendamustine hydrochloride Form 2, bendamustine hydrochloride Form 3, bendamustine hydrochloride Form 4, amorphous bendamustine hydrochloride or a mixture thereof, processes for their preparation and lyophilized composition comprising the solid forms. According to the disclosure, monohydrate of bendamustine hydrochloride has been prepared previously. The monohydrate has a reported melting point of 152-156°C which is similar to that of the observed melting point of bendamustine hydrochloride Form 2.
It is known that synthetic compounds can contain extraneous compounds or impurities resulting from their synthesis or degradation. The impurities can be unreacted starting materials, by-products of the reaction, products of side reactions, or degradation products. Generally, impurities in an active pharmaceutical ingredient (API) may arise from degradation of the API itself, or during the preparation of the API. Impurities in Bendamustine hydrochloride or any active pharmaceutical ingredient (API) are undesirable and might be harmful.
Regulatory authorities worldwide require that drug manufacturers isolate, identify and characterize the impurities in their products. Furthermore, it is required to control the levels of these impurities in the final drug compound obtained by the manufacturing process and to ensure that the impurity is present in the lowest possible levels, even if structural determination is not possible. The product mixture of a chemical reaction is rarely a single compound with sufficient purity to comply with pharmaceutical standards. Side products and byproducts of the reaction and adjunct reagents used in the reaction will, in most cases, also be present in the product mixture. At certain stages during processing of the active pharmaceutical ingredient, the product is analyzed for purity, typically, by HPLC, TLC. or GC analysis, to determine if it is suitable for continued processing and, ultimately, for use in a pharmaceutical product. Purity standards are set with the intention of ensuring that an API is as free of impurities as possible, and, thus, are as safe as possible for clinical use. The United States Food and Drug Administration guidelines recommend that the amounts of some impurities are limited to less than 0.1 percent.
Generally, impurities are identified spectroscopically and by other physical methods, and then the impurities are associated with a peak position in a chromatogram (or a spot on a TLC plate). Thereafter, the impurity can be identified by its position in the chromatogram, which is conventionally measured in minutes between injection of the sample on the column and elution of the particular component through the detector, known as the “retention time” (“RT”). This time period varies daily based upon the condition of the instrumentation and many other factors. To mitigate the effect that such variations have upon accurate identification of an impurity, practitioners use “relative retention time” (“RRT”) to identify impurities. The RRT of an impurity is its retention time divided by the retention time of a reference marker.
It is known by those skilled in the art, the management of process impurities is greatly enhanced by understanding their chemical structures and synthetic pathways, and by identifying the parameters that influence the amount of impurities in the final product.
Therefore, there remains a need for improved process for the preparation of bendamustine hydrochloride, producing high yield and purity, and well-suited for use on an industrial scale. Despite the existence of various polymorphic forms of bendamustine hydrochloride, there exists a need for a simple process for the preparation of the stable form of bendamustine hydrochloride which is amenable to scale up and results in high yield and purity.
Bendamustine (INN, trade names Treakisym, Ribomustin, Levact and Treanda; also known as SDX-105) is a nitrogen mustardused in the treatment of chronic lymphocytic leukemia[1] and lymphomas. It belongs to the family of drugs called alkylating agents. It is also being studied for the treatment of sarcoma.[2] It is also being investigated in phase II trials for the non-cancer treatment of AL Amyloidosis.
Bendamustine hydrochloride, initially synthesized in 1963 in the German Democratic Republic, is an alkylating agent that has been shown to have therapeutic utility in treating diseases such as chronic lymphocytic leukemia, Hodgkin’s disease, non-Hodgkin’s lymphoma, multiple myeloma, and breast cancer.It was available from 1971 to 1992 under the trade name Cytostasanand, since that time, has been marketed in Germany as Ribomustin.In March 2008 the FDA approved bendamustine hydrochloride under the trade name Treanda for the treatment of chronic lymphocytic leukemia (CLL). Approval for use in indolent B-cell non-Hodgkin’s lymphoma (NHL) was received in 2009.
History
Bendamustine was first synthesized in 1963 by Ozegowski and Krebs in East Germany (the former German Democratic Republic). Until 1990 it was available only in East Germany. East German investigators found that it was useful for treating chronic lymphocytic leukemia, Hodgkin’s disease, non-Hodgkin’s lymphoma, multiple myeloma and lung cancer.
Bendamustine received its first marketing approval in Germany, where it is marketed under the tradename Ribomustin, by Astellas Pharma GmbH’s licensee, Mundipharma International Corporation Limited. It is indicated as a single-agent or in combination with other anti-cancer agents for indolent non-Hodgkin’s lymphoma, multiple myeloma, and chronic lymphocytic leukemia. SymBio Pharmaceuticals Ltd holds exclusive rights to develop and market bendamustine HCl in Japan and selected Asia Pacific Rim countries.
In March 2008, Cephalon received approval from the United States Food and Drug Administration to market bendamustine in the US, where it is sold under the tradename Treanda, for treatment of chronic lymphocytic leukemia.[3]
In October 2008, the FDA granted further approval to market Treanda for the treatment of indolent B-cell non-Hodgkin’s lymphoma that has progressed during or within six months of treatment with rituximab or a rituximab-containing regimen.[4]
Pharmacology
Bendamustine is a white, water soluble microcrystalline powder with amphoteric properties. It acts as an alkylating agent causing intra-strand and inter-strand cross-links between DNA bases.
After intravenous infusion it is extensively metabolised in the liver by cytochrome p450. More than 95% of the drug is bound to protein – primarily albumin. Only free bendamustine is active. Elimination is biphasic with a half-life of 6–10 minutes and a terminal half-life of approximately 30 minutes. It is eliminated primarily through the kidneys. This paragraph is inconsistent with sidebar for primary excretion pathway.
Chemotherapeutic uses
Bendamustine has been used both as sole therapy and in combination with other agents including etoposide, fludarabine,mitoxantrone, methotrexate, prednisone, rituximab, vincristine and 90Y-ibritumomab tiuxetan.
Lymphomas
One combination for stage III/IV relapsed or refractory indolent lymphomas and mantle cell lymphoma (MCL), with or without prior rituximab-containing chemoimmunotherapy treatment, is bendamustine with mitoxantrone and rituximab.[5] In Germany in 2012 it has become the first line treatment of choice for indolent lymphoma.[6] after Trial results released in June 2012 showed that it more than doubled disease progression-free survival when given along with rituximab. The combination also left patients with fewer side effects than the older R-CHOP treatment.[7]
Adverse effects
Common adverse reactions are typical for the class of nitrogen mustards, and include nausea, fatigue, vomiting, diarrhea, fever, constipation, loss of appetite, cough, headache, unintentional weight loss, difficulty breathing, rashes, and stomatitis, as well as immunosuppression, anemia, and low platelet counts. Notably, this drug has a low incidence of hair loss (alopecia) unlike most other chemotherapy drugs.[8]
……………………
http://www.google.com/patents/WO2013046223A1?cl=en
First aspect of the present invention provides an improved process for the preparation of Bendamustine hydrochloride of the formula (VI)
comprising the steps of:
a) reacting a compound of the formula (II), wherein R is Ci-C6 alkyl
with a 2-haloethanol in the presence of a base to give a compound of formula (III);
b) reacting the compound of formula (III) with a chlorinating agent to provide a compound of formula (IV);
c) hydrolyzing the compound of formula (IV) with Lithium source to give a compound of formula (V); and
d) converting the compound of formula (V) to bendamustine or bendamustine hydrochloride of Formula VI .
Reference Example- 1
Preparation of Bendamustine Hydrochloride as per Patent No. DD159877
Ethyl 4-[l-methyl-5-bis-(2-hydroxyethyl)-amino-benzimidazolyl- 2]butanoate (4, 4.305g) was added to chloroform (36mL) and agitated till clear solution is formed. The solution was cooled to 0°C. Thionyl chloride (2.175g) was added to the above solution within 40 minutes maintaining the temperature of the solution to 0-5°C by cooling. The reaction mixture was agitated at 0-5°C for 1 hour. The temperature was raised slowly to room temperature by removing cooling within 2.5 to 3 hrs and subsequently agitated at room temperature for 15 to 16 hrs. The solution was dispersed by agitating in 37.5mL concentrated hydrochloric acid whereby the excessive thionyl chloride was decomposed under increased hydrochloric acid and S02development. The chloroform was distilled away and further stirred for 3 hrs at around 95°C. Activated carbon (0.78g) was added to the solution and stirred for further 30 minutes at around 95 °C. The solution was concentrated to almost 8mL under vacuum and the residue was diluted with 24mL of water and stirred up to crystallization. The further purification was done by recrystallization from water.
Example-4
Preparation of Bendamustine hydrochloride (VI) through Lithium 4-[l-methyl-5- bis-(2-chloroethyl)-benzimidazoIyl-2] butanoate (V)
Activated charcoal (11. Og) was added to Cone. HC1 (165.0 mL) under stirring and cooled to 5-10°C. Lithium 4-[l-methyl-5-bis-(2-chloroethyl)- benzimidazolyl-2] butanoate (V, HO.Og, 0.302 mol) was added below 65°C under agitation and agitated for 30-45 minutes. The reaction mass was filtered on celite bed prewashed with cone. HC1 and the celite bed was washed with cone. HC1 (27.5mL). The filtrate and washings were combined. DM water (550.0mL) was added to combined filtrate and washings and agitated for 15 minutes. DM water (1.1L) was added and stirred at 20-30°C for 30 minutes. The resulting mass was cooled to 0-5°C and maintained at a temperature of 0 to 5°C for 30 minutes under agitation. The solid was filtered, washed with chilled (0-5°C) DM water twice (220.0 mL each X 2 = 440.0mL) followed by with chilled acetone (0-5°C) (55. OmL) and sucked dried for 1 hour. The solid cake was agitated with acetone (1 lOO.OmL) for 10 minutes and filtered. The solid material was dried at 20-25°C under 100-200 mbar vacuum for one hour till moisture content is between 4.4-6.0% w/w to give the title compound (VI, 80.0g; 67.10%), with a purity of 99.86%.
…………………………..

Gao, L.; Wang, Y.; Song, D. Chinese J. New Drugs 2007, 16, 1960
Ozegowski, V. W.; Krebs, D. J. Prakt. Chem. 1963, 20, 178
Werner, W.; Letsch, G.; Ihn, W.; Sohr, R.; Preiss, R. Pharmazie 1991, 46, 113
Ozegowski, W.; Krebs, D. J. Prakt. Chem. 1963, 20, 178
Werner, W.; Letsch, G.; Ihn, W. Pharmazie 1987,42, 272
………………………………..

-
(a) Chen, J., Przyuski, K., and Roemmele, R. U.S. Patent 8,420,829, April 16, 2013;
Chem. Abstr. 2010, 152, 454105.
(b) Chen, J.; Przyuski, K.; Roemmele, R.; Bakale, R. P.Org. Process Res. Dev. 2011, 15, 1063………………………………………Org. Process Res. Dev., 2011, 15 (5), pp 1063–1072DOI: 10.1021/op200176f![Abstract Image]() Process Research and Development activities leading to a new and efficient route to bendamustine hydrochloride, 1, the active ingredient in Treanda, a treatment for blood cancers, are disclosed. Two key features of this new process include a one-pot hydrogenation/dehydration sequence to construct the benzimidazole moiety and a novel reductive alkylation using chloroacetic acid and borane to install the bischloroethyl side chain. The number of synthetic steps has been significantly reduced to five from the eight in the current commercial process. The overall yield has been improved from 12% to 45%. Additionally, this new route eliminates chloroform, ethylene oxide, and sodium sulfide. Scale-up of the new route has been successfully demonstrated to prepare kilogram quantities of bendamustine hydrochloride.…………………………Org. Process Res. Dev., 2011, 15 (5), pp 1063–1072DOI: 10.1021/op200176f
Process Research and Development activities leading to a new and efficient route to bendamustine hydrochloride, 1, the active ingredient in Treanda, a treatment for blood cancers, are disclosed. Two key features of this new process include a one-pot hydrogenation/dehydration sequence to construct the benzimidazole moiety and a novel reductive alkylation using chloroacetic acid and borane to install the bischloroethyl side chain. The number of synthetic steps has been significantly reduced to five from the eight in the current commercial process. The overall yield has been improved from 12% to 45%. Additionally, this new route eliminates chloroform, ethylene oxide, and sodium sulfide. Scale-up of the new route has been successfully demonstrated to prepare kilogram quantities of bendamustine hydrochloride.…………………………Org. Process Res. Dev., 2011, 15 (5), pp 1063–1072DOI: 10.1021/op200176fPreparation of Bendamustine Hydrochloride (1)
A……../…………..purity of 99.9 A%.1H NMR (400 MHz, DMSO-d6) δ 12.3 (br s, 1H), 7.72 (d, J = 9.3 Hz, 1H), 7.14 (d, J = 2.3 Hz, 1H), 6.89 (dd, J = 9.3, 2.3 Hz, 1H), 3.90 (s, 3H), 3.80 (m, 8H), 3.14 (t, J = 7.6 Hz, 2H), 2.42 (t, J = 7.2 Hz, 2H), 2.01 (quint, J = 7.6 Hz, 2H);LC/MS (ESI, m/z) 358.2 Da (M + 1).……………………..Bendamustine, 4-[5-[bis(2-chloroethyl)amino]-l-methyl-2-benzimidazolyl]butyric acid of formula (1)
, is a cytostatic agent currently approved, in a form of a hydrochloride salt, for treatment of various cancer diseases, e.g. chronic lymphocytic leukemia. It is marketed in the form of a lyophilized powder for intravenous injection, e.g., under the brand name Ribomustin.
Bendamustine, including bendamustine hydrochloride, was first disclosed in DD 34727. Bendamustine hydrochloride may exist, in solid state, in various polymorphic forms, which are disclosed, e.g. in WO 2009/120386. The hydrochloride product disclosed in DD 34727 is a monohydrate. The original process for making bendamustine in DD 34727 comprises the following synthetic pathway:
The group R in the above process is an ethyl group.
The last step of the above process was subsequently technologically improved in DD 159877.
Without providing any experimental detail, DD 34727 also teaches that the starting compound of formula (4) for the above process may be prepared from 2-methylamino-5-nitro- aniline of formula (2) and glutaric acid anhydride. The obtained anilide of formula (3) is cyclized in diluted hydrochloric acid.
Li-Mei et al, in Zhongguo Xinyao Zazhi, Chinese Journal of New Drugs (2007), 16(23), 1960-1961, disclose a process for the preparation of bendamustine hydrochloride in a total yield of 33.5%, which also involves reacting the compound of formula (10) with ethylene oxide to give compound (11). Starting from 2,4-dinitro-l-chlorobenzene, compound (11) is obtained in an overall yield of about 40%.IP.com Journal 2009, 9(7B), 21 discloses a process for the preparation of ethyl-4-[5-[bis(2- hydroxyethyl)amino]-l-methylbenzimidazol-2-yl]butanoate (11) [R=Et], wherein the corresponding compound of formula (10) reacts, instead of ethylene oxide, with 2-halo ethanol in the presence of an inorganic base.
A similar process has been disclosed in WO 2011/079193, wherein the base employed in the reaction of the compound of formula (10) with the 2-haloethanol is an organic base, which is advantageous over inorganic base. The preferred ester group R in the compounds (10) and (11) is the 2-propyl group.
WO 2010/042568 discloses a second basic process for making bendamustine, which is based on providing the compound of formula (5)
(5)
, wherein R is typically a methyl group, by a two step synthesis starting from 2,4- dinitroaniline of formula (6) via the dinitroanilide of formula (7)
This compound of formula (5) is subjected, at reductive conditions (preferably hydrogenation over a platinum catalyst), to a cyclization reaction forming a compound of formula (8)
(8)
, which subsequently may be dehydrated by a strong acid to yield the compound of formula
(10) above. The substituent R in both formulas is a methyl group.
The compound of formula (10) is advantageously subjected to a reductive alkylation with a chloroacetic acid or chloroacetylaldehyde. The reductive agent in the alkylation is suitably a borane or a borohydride. This way, the bendamustine ester of formula (la)
, wherein R is a methyl group, is made directly, without need of forming an intermediate bis-hydroxyethyl compound (11). In the last step of the overall process, the ester (la) is hydrolyzed by a strong acid.
In any process of making bendamustine, various impurities are formed due to various reactive groups in the molecule.
The subject of the present invention is a novel synthetic route to intermediates involved in the synthesis of bendamustine of formula (1) as well as of salts and esters thereof. The approach is based on a novel use of a compound of formula (13) below as the starting material in a synthetic transformation leading to bendamustine, or a pharmaceutically acceptable salt thereof.
In a first aspect, the invention provides a process for making a compound of formula (11), or a salt thereof,
wherein R is hydrogen or a C1-C4 alkyl group,
said process comprising the following steps:
a] providing the compound of formula (13), preferably by reaction of the compound of formula (12) with methylamine,
(12) (13)b] reduction of the compound of formula (13), preferably by hydrogen under catalysis by a transition metal, to an amino compound of formula (14),
c] condensation of the compound of formula (14) with glutaric acid anhydride, or a functional analogue thereof, providing a tertiary alcohol compound of formula (15)
and/or any tautomeric forms thereof according to formula (14A) or (14B)
(14A), (14B),
d] dehydratation and, optionally, esterification of the product of the step c) , preferably in the presence of a strong acid, to yield the compound of formula (11).
In a particular aspect, the above process sequence leading to a compound of formula (11) further comprises a subsequent step of converting the compound of formula (11) to
bendamustine, a salt thereof or an ester thereof, conventionally by reaction with thionyl chloride, followed by ester hydrolysis and salt formation using hydrochloric acid. In yet another aspect, the process sequence of the above steps a) to c) or, optionally, of the above steps a) to d), is performed without isolation or purification of intermediates.
The compounds of formula (14), (14A), (14B) and (15), the above processes of making them, and the use thereof as a starting material for making compounds of formula (11) and/or bendamustine of formula (1), or a pharmaceutically acceptable salt thereof, form next particular aspects of the present invention.
![Figure imgf000010_0001]()
Example 3
A solution of [11, R = Me] (4.0 g, 12 mmol) in dichloromethane (40.0ml) was prepared.
A 100 ml, three -necked, round -bottomed flask equipped with a magnetic stirring bar and a reflux condenser was charged with thionyl chloride (3.60 g, 2.20 ml, 30 mmol) and dichloromethane (12.0 ml) to produce a clear solution. The latter was stirred at 500 rpm at 23 °C and the solution of [11 , R = Me] was added over 15 min via a syringe pump. The resulting mixture was stirred at 500 rpm at 23 °C for 15 min and then at 35 °C for 3.0 h. An aqueous solution of hydrochloric acid (19.4 %) was prepared by mixing of concentrated hydrochloric acid (4.7 g, 4.0 ml) with water (4.0 g, 4.0 ml) and the solution was charged to the reaction mixture. The mixture was further stirred at 500 rpm at 60 °C for 2.0 h under reduced pressure of 100 mbar and the escaping volatiles were condensed and collected outside the reaction vessel. An aqueous solution of hydrochloric acid (4.0 ml, 5 M) was charged in order to dilute the reaction mixture. The mixture was filtered through diatomaceous earth and the filter cake was washed with aqueous solution of hydrochloric acid (2x 1.0 ml, 5 M). The collected filtrate was treated with activated carbon, the used carbon was filtered off, washed with aqueous solution of hydrochloric acid (2x 1.0 ml, 5M), and the filtrate was collected to a 100 ml, round -bottomed flask equipped with a magnetic stirring bar. The filtrate was stirred and diluted with water (40.0 g, 40.0 ml). The slurry was filtered and the filter cake was washed with water (2x 1.0 ml). The filter cake (3.8 g) was charged to a 25 ml, round-bottomed flask equipped with a magnetic stirring bar containing an aqueous solution of hydrochloric acid (8.5 ml, 5M). The mixture was stirred at 60 °C until the solids were dissolved and activated carbon (0.38 g) was charged. The mixture was stirred at 40 °C for additional 5 min and the suspension was filtered through a diatomaceous earth pad. The filter cake was washed with aqueous solution of hydrochloric acid (2x 1.0 ml, 5M) and the filtrate was collected to a 100 ml, round -bottomed equipped with a magnetic stirring bar. The filtrate was stirred at 23 °C and water (42.0 g, 42.0 ml) was added and the mixture was stirred at 23 °C for additional 1 h. The slurry of crystals was filtered and the filter cake was washed with aqueous solution of hydrochloric acid (2x 3.0 ml, 5M). The filtrate was discarded and the filter cake was dried to produce Bendamustine hydrochloride monohydrate (3.3 g) with an overall isolated yield of 67 % and with a chemical purity of 99.9 % by HPLC peak area normalization.
Characteriz ation
lU NMR (400 MHz, DMSO-J<5): δ (ppm) = 2.05 (q, J = 7.51 Hz, 2H), 2.41 (t, J = 7.19 Hz, 2H), 3.18 (t, / = 7.63 Hz, 2H), 3.79 (m, 8H), 3.90 (s, 3H), 6.95 (d, J = 2.31 Hz, 1H), 7.11 (dd, Jx = 2.40 Hz, J2 = 9.20 Hz, 1H), 7.80 (d, J = 9.20 Hz, 1H).
Assay H20 (Karl-Fisher titration): 4.7 %
Assay HC1 (argentometric titration): 8.8 %
Bendamustine ![Bendamustine.png]()
Systematic (IUPAC) name 4-[5-[Bis(2-chloroethyl)amino]-1-methylbenzimidazol-2-yl]butanoic acid Clinical data Trade names Treanda AHFS/Drugs.com Consumer Drug Information MedlinePlus a608034 Licence data US FDA:link Pregnancy cat. - US: D
Legal status Routes Intravenous infusion Pharmacokinetic data Bioavailability NA (intravenous only) Protein binding 94–96% Metabolism Hydrolyzed to inactive metabolites. Two minor metabolites (M3 and M4) formed by CYP1A2 Half-life 40 min (bendamustine), 3 h (M3), 30 min (M4) Excretion Mostly fecal Identifiers CAS number 16506-27-7 ![Yes]()
ATC code L01AA09 PubChem CID 65628 ChemSpider 59069 ![Yes]()
UNII 9266D9P3PQ ![Yes]()
ChEMBL CHEMBL487253 ![Yes]()
Chemical data Formula C16H21Cl2N3O2 Mol. mass 358.262 g/mol References
- Kath R, Blumenstengel K, Fricke HJ, Höffken K (January 2001). “Bendamustine monotherapy in advanced and refractory chronic lymphocytic leukemia”. J. Cancer Res. Clin. Oncol. 127(1): 48–54. doi:10.1007/s004320000180. PMID 11206271.
- Bagchi S (August 2007). “Bendamustine for advanced sarcoma”. Lancet Oncol. 8 (8): 674. doi:10.1016/S1470-2045(07)70225-5. PMID 17726779.
- “Cephalon press release – Cephalon Receives FDA Approval for TREANDA, a Novel Chemotherapy for Chronic Lymphocytic Leukemia”. Retrieved 2008-03-23.
- “Cephalon press release -Cephalon Receives FDA Approval for TREANDA to Treat Patients with Relapsed Indolent Non-Hodgkin’s Lymphoma”. Retrieved 2008-11-03.
- Weide R, Hess G, Köppler H, et al. (2007). “High anti–lymphoma activity of bendamustine/mitoxantrone/rituximab in rituximab pretreated relapsed or refractory indolent lymphomas and mantle cell lymphomas. A muticenter phase II study of the German Low Grade Lymphoma Study Group (GLSG)”. Leuk. Lymphoma. 48 (7): 1299–1306. doi:10.1080/10428190701361828.PMID 17613757.
- New Combo Replaces CHOP for Lymphoma. Dec 2012
- “‘Rediscovered’ Lymphoma Drug Helps Double Survival: Study”. June 3, 2012.
- Tageja, Nishant; Nagi, Jasdeepa; “Bendamustine: something old, something new”; Cancer Chemotherapy and Pharmacology, 2010 Aug;66(3):413-23. doi: 10.1007/s00280-010-1317-x.
External links
- Manufacturer’s official website intended for US patients
References:Bifunctional alkylating agent. Prepn: W. Ozegowski, D. Krebs, J. Prakt. Chem. 20, 178 (1963); eidem,Zentralbl. Pharm. Pharmakother. Laboratoriumsdiagn. 110, 1013 (1971). Antitumor activity: W. Jungstand et al., ibid. 1021.Capillary GC determn in plasma: H. Weber et al., J. Chromatogr. 525, 459 (1990).Toxicity study: U. Horn et al., Arch. Toxicol.Suppl. 8, 504 (1985).Clinical evaluation in non-Hodgkin’s lymphomas: K. Bremer, J. Cancer Res. Clin. Oncol. 128, 603 (2002); in chronic lymphocytic leukemia: T. Lissitchkov et al., ibid. 132, 99 (2006); with prednisone in multiple myeloma: W. Pönisch et al.,ibid 205.Review of pharmacology and clinical development: K. Bremer, W. Roth, Tumordiagn. Ther. 17, 1-6 (1996); J. A. Barman Balfour, K. L. Goa, Drugs 61, 631-638 (2001).WO2009120386A2 Mar 26, 2009 Oct 1, 2009 Cephalon, Inc. Novel solid forms of bendamustine hydrochloride WO2010036702A1 Sep 23, 2009 Apr 1, 2010 Cephalon, Inc. Liquid formulations of bendamustine WO2010042568A1 Oct 7, 2009 Apr 15, 2010 Cephalon, Inc. Processes for the preparation of bendamustine WO2011079193A2 Dec 22, 2010 Jun 30, 2011 Dr. Reddy’s Laboratories Ltd. Preparation of bendamustine and its salts DD34727A Title not available DD159877A1 Title not available US20060128777 Nov 4, 2005 Jun 15, 2006 Bendall Heather H Cancer treatments US20060159713 Jan 12, 2006 Jul 20, 2006 Cephalon, Inc. Bendamustine pharmaceutical compositions
Filed under: Uncategorized Tagged: bendamustine, Bendamustine hydrochloride