![Figure imgf000005_0001]() INCB-39110,
INCB-39110,
CAS 1334298-90-6
INCB-039110, Jak1 tyrosine kinase inhibitor
- 3-Azetidineacetonitrile, 1-[1-[[3-fluoro-2-(trifluoromethyl)-4-pyridinyl]carbonyl]-4-piperidinyl]-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]-
C26H23F4N9O (MW, 553.51)
{ l- { l-[3-fluoro-2- (trifluoromethyl)isonicotinoyl]piperidin-4-yl}-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4- yl)-lH-pyrazol-l-yl]azetidin-3-yl}acetonitrile
2-(3-(4-(7H-pyrrolo[2,3-( Jpyrimidin-4-yl)-lH- pyrazol- 1 -yl)- 1 -( 1 -(3 -fluoro-2-(trifluoromethyl)isonicotinoyl)piperidin-4-yl)azetidin- 3-yl)acetonitrile
2-(3-(4-(7H- Pyrrolo[2,3 -i/]pyrimidin-4-yl)- lH-pyrazol- 1 -yl)- 1 -(1 -(3 -fluoro-2- (trifluoromethyl)isonicotinoyl)piperidin-4-yl)azetidin-3-yl)acetonitrile adipate MAY BE THE DRUG… HAS CAS 1334302-63-4
![Figure imgf000005_0001]()
![Adipic acid]() ADIPATE OF INCB-39110
ADIPATE OF INCB-39110
ALSO/OR
![Figure US20130060026A1-20130307-C00027]()
3-Azetidineacetonitrile, 1-[1-(3-fluorobenzoyl)-4-methyl-4-piperidinyl]-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]-, 2,2,2-trifluoroacetateMAY BE THE DRUG ????… HAS CAS 1334300-52-5
US 2011/0224190
![]()
IN PHASE 2 for the treatment of rheumatoid arthritis, myelofibrosis, rheumatoid arthritis and plaque psoriasis.
SEE
http://clinicaltrials.gov/show/NCT01633372
Jak2 tyrosine kinase inhibitor; Jak1 tyrosine kinase inhibitor
Breast tumor; Chronic obstructive pulmonary disease; Crohns disease; Inflammatory bowel disease; Influenza virus infection; Insulin dependent diabetes; Liver tumor; Multiple sclerosis; Prostate tumor; Rheumatoid arthritis; SARS coronavirus infection
Used for treating cancers (eg prostate cancer, hepatic cancer and pancreatic cancer) and autoimmune diseases. Follows on from WO2013036611, claiming the process for preparing the same JAK inhibitor. Incyte is developing INCB-39110 (phase II, September 2014), for the oral treatment of myelofibrosis, hematological neoplasm and non-small cell lung cancer.
INCB-039110 is a Jak1 inhibitor in phase II clinical studies at Incyte for the treatment of rheumatoid arthritis, myelofibrosis, rheumatoid arthritis and plaque psoriasis. The company is also conducting a phase I clinical study for the treatment of advanced or metastatic solid tumors.
Protein kinases (PKs) regulate divINCB-039110 is a Jak1 inhibitor in phase II clinical studies at Incyte for the treatment of rheumatoid arthritis, myelofibrosis, rheumatoid arthritis and plaque psoriasis. The company is also conducting a phase I clinical study for the treatment of advanced or metastatic solid tumors.erse biological processes including cell growth, survival, differentiation, organ formation, morphogenesis, neovascularization, tissue repair, and regeneration, among others. Protein kinases also play specialized roles in a host of human diseases including cancer. Cytokines, low-molecular weight polypeptides or glycoproteins, regulate many pathways involved in the host
inflammatory response to sepsis. Cytokines influence cell differentiation,
proliferation and activation, and can modulate both pro-inflammatory and antiinflammatory responses to allow the host to react appropriately to pathogens.
Signaling of a wide range of cytokines involves the Janus kinase family (JAKs) of protein tyrosine kinases and Signal Transducers and Activators of Transcription
(STATs). There are four known mammalian JAKs: JAK1 (Janus kinase-1), JAK2, JAK3 (also known as Janus kinase, leukocyte; JAKL; and L-JAK), and TYK2
(protein-tyros ine kinase 2).
Cytokine-stimulated immune and inflammatory responses contribute to pathogenesis of diseases: pathologies such as severe combined immunodeficiency (SCID) arise from suppression of the immune system, while a hyperactive or inappropriate immune/inflammatory response contributes to the pathology of autoimmune diseases (e.g., asthma, systemic lupus erythematosus, thyroiditis, 20443-0253WO1 (INCY0124-WO1) PATENT myocarditis), and illnesses such as scleroderma and osteoarthritis (Ortmann, R. A., T. Cheng, et al. (2000) Arthritis Res 2(1): 16-32).
Deficiencies in expression of JAKs are associated with many disease states. For example, Jakl-/- mice are runted at birth, fail to nurse, and die perinatally (Rodig, S. J., M. A. Meraz, et al. (1998) Cell 93(3): 373-83). Jak2-/- mouse embryos are anemic and die around day 12.5 postcoitum due to the absence of definitive
erythropoiesis.
The JAK/STAT pathway, and in particular all four JAKs, are believed to play a role in the pathogenesis of asthmatic response, chronic obstructive pulmonary disease, bronchitis, and other related inflammatory diseases of the lower respiratory tract. Multiple cytokines that signal through JAKs have been linked to inflammatory diseases/conditions of the upper respiratory tract, such as those affecting the nose and sinuses (e.g., rhinitis and sinusitis) whether classically allergic reactions or not. The JAK/STAT pathway has also been implicated in inflammatory diseases/conditions of the eye and chronic allergic responses.
Activation of JAK/STAT in cancers may occur by cytokine stimulation (e.g. IL-6 or GM-CSF) or by a reduction in the endogenous suppressors of JAK signaling such as SOCS (suppressor or cytokine signaling) or PIAS (protein inhibitor of activated STAT) (Boudny, V., and Kovarik, J., Neoplasm. 49:349-355, 2002).
Activation of STAT signaling, as well as other pathways downstream of JAKs (e.g., Akt), has been correlated with poor prognosis in many cancer types (Bowman, T., et al. Oncogene 19:2474-2488, 2000). Elevated levels of circulating cytokines that signal through JAK/STAT play a causal role in cachexia and/or chronic fatigue. As such, JAK inhibition may be beneficial to cancer patients for reasons that extend beyond potential anti-tumor activity.
JAK2 tyrosine kinase can be beneficial for patients with myeloproliferative disorders, e.g., polycythemia vera (PV), essential thrombocythemia (ET), myeloid metaplasia with myelofibrosis (MMM) (Levin, et al, Cancer Cell, vol. 7, 2005: 387- 397). Inhibition of the JAK2V617F kinase decreases proliferation of hematopoietic cells, suggesting JAK2 as a potential target for pharmacologic inhibition in patients with PV, ET, and MMM. 20443-0253WO1 (INCY0124-WO1) PATENT
Inhibition of the JAKs may benefit patients suffering from skin immune disorders such as psoriasis, and skin sensitization. The maintenance of psoriasis is believed to depend on a number of inflammatory cytokines in addition to various chemokines and growth factors (JCI, 1 13 : 1664-1675), many of which signal through JAKs (Adv Pharmacol. 2000;47: 113-74).
JAKl plays a central role in a number of cytokine and growth factor signaling pathways that, when dysregulated, can result in or contribute to disease states. For example, IL-6 levels are elevated in rheumatoid arthritis, a disease in which it has been suggested to have detrimental effects (Fonesca, J.E. et al, Autoimmunity
Reviews, 8:538-42, 2009). Because IL-6 signals, at least in part, through JAKl, antagonizing IL-6 directly or indirectly through JAKl inhibition is expected to provide clinical benefit (Guschin, D., N., et al Embo J 14: 1421, 1995; Smolen, J. S., et al. Lancet 371 :987, 2008). Moreover, in some cancers JAKl is mutated resulting in constitutive undesirable tumor cell growth and survival (Mullighan CG, Proc Natl Acad Sci U S A.106:9414-8, 2009; Flex E., et al.J Exp Med. 205:751-8, 2008). In other autoimmune diseases and cancers elevated systemic levels of inflammatory cytokines that activate JAKl may also contribute to the disease and/or associated symptoms. Therefore, patients with such diseases may benefit from JAKl inhibition. Selective inhibitors of JAKl may be efficacious while avoiding unnecessary and potentially undesirable effects of inhibiting other JAK kinases.
Selective inhibitors of JAKl, relative to other JAK kinases, may have multiple therapeutic advantages over less selective inhibitors. With respect to selectivity against JAK2, a number of important cytokines and growth factors signal through JAK2 including, for example, erythropoietin (Epo) and thrombopoietin (Tpo)
(Parganas E, et al. Cell. 93:385-95, 1998). Epo is a key growth factor for red blood cells production; hence a paucity of Epo-dependent signaling can result in reduced numbers of red blood cells and anemia (Kaushansky K, NEJM 354:2034-45, 2006). Tpo, another example of a JAK2-dependent growth factor, plays a central role in controlling the proliferation and maturation of megakaryocytes – the cells from which platelets are produced (Kaushansky K, NEJM 354:2034-45, 2006). As such, reduced Tpo signaling would decrease megakaryocyte numbers (megakaryocytopenia) and lower circulating platelet counts (thrombocytopenia). This can result in undesirable 20443-0253WO1 (INCY0124-WO1) PATENT and/or uncontrollable bleeding. Reduced inhibition of other JAKs, such as JAK3 and Tyk2, may also be desirable as humans lacking functional version of these kinases have been shown to suffer from numerous maladies such as severe-combined immunodeficiency or hyperimmunoglobulin E syndrome (Minegishi, Y, et al.
Immunity 25:745-55, 2006; Macchi P, et al. Nature. 377:65-8, 1995). Therefore a JAK1 inhibitor with reduced affinity for other JAKs would have significant
advantages over a less-selective inhibitor with respect to reduced side effects involving immune suppression, anemia and thrombocytopenia.
……………………….
http://www.google.com/patents/US20110224190
EXAMPLESThe example compounds below containing one or more chiral centers were obtained in enantiomerically pure form or as scalemic mixtures, unless otherwise specified.Unless otherwise indicated, the example compounds were purified by preparativeHPLC using acidic conditions (method A) and were obtained as a TFA salt or using basic conditions (method B) and were obtained as a free base.Method A:Column: Waters Sun Fire C18, 5 μm particle size, 30×100 mm;
Mobile phase: water (0.1% TFA)/acetonitrile
Flow rate: 60 mL/min
Gradient: 5 min or 12 min from 5% acetonitrile/95% water to 100% acetonitrileMethod B:Column: Waters X Bridge C18, 5 μm particle size, 30×100 mm;
Mobile phase: water (0.15% NH
4OH)/acetonitrileMethod C:Column: C18 column, 5 μm OBD
Mobile phase: water+0.05% NH
4OH (A), CH
3CN+0.05% NH
4OH (B)Gradient: 5% B to 100% B in 15 minFlow rate: 60 mL/minExample 1
{1-{1-[3-Fluoro-2-(trifluoromethyl)isonicotinoyl]piperidin-4-yl}-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]azetidin-3-yl}acetonitrile
Step A: tert-Butyl 3-Oxoazetidine-1-carboxylate
To a mixture of tert-butyl 3-hydroxyazetidine-1-carboxylate (10.0 g, 57.7 mmol), dimethyl sulfoxide (24.0 mL, 338 mmol), triethylamine (40 mL, 300 mmol) and methylene chloride (2.0 mL) was added sulfur trioxide-pyridine complex (40 g, 200 mmol) portionwise at 0° C. The mixture was stirred for 3 hours, quenched with brine, and extracted with methylene chloride. The combined extracts were dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by silica gel column (0-6% ethyl acetate (EtOAc) in hexanes) to give tert-butyl 3-oxoazetidine-1-carboxylate (5.1 g, 52% yield).
Step B: tert-Butyl 3-(Cyanomethylene)azetidine-1-carboxylate
![Figure US20110224190A1-20110915-C00027]()
An oven-dried 1 L 4-neck round bottom flask fitted with stir bar, septa, nitrogen inlet, 250 ml addition funnel and thermocouple was charged with sodium hydride (5.6 g, 0.14 mol) and tetrahydrofuran (THF) (140 mL) under a nitrogen atmosphere. The mixture was chilled to 3° C., and then charged with diethyl cyanomethylphosphonate (22.4 mL, 0.138 mol) dropwise via a syringe over 20 minutes. The solution became a light yellow slurry. The reaction was then stirred for 75 minutes while warming to 18.2° C. A solution of tert-butyl 3-oxoazetidine-1-carboxylate (20 g, 0.1 mol) in tetrahydrofuran (280 mL) was prepared in an oven-dried round bottom, charged to the addition funnel via canula, then added to the reaction mixture dropwise over 25 minutes. The reaction solution became red in color. The reaction was allowed to stir overnight. The reaction was checked after 24 hours by TLC (70% hexane/EtOAc) and found to be complete. The reaction was diluted with 200 mL of 20% brine and 250 mL of EtOAc. The solution was partitioned and the aqueous phase was extracted with 250 mL of EtOAc. The combined organic phase was dried over MgSO4 and filtered, evaporated under reduced pressure, and purified by flash chromatography (0% to 20% EtOAc/hexanes, 150 g flash column) to give the desired product, tert-butyl 3-(cyanomethylene)azetidine-1-carboxylate (15 g, 66.1% yield).
Step C: 4-Chloro-7-{[2-(trimethylsilyl)ethoxy]methyl}-7H-pyrrolo[2,3-d]pyrimidine
![Figure US20110224190A1-20110915-C00028]()
To a suspension of sodium hydride (36.141 g, 903.62 mmol) in N,N-dimethylacetamide (118 mL) at −5° C. (ice/salt bath) was added a dark solution of 4-chloropyrrolo[2,3-d]pyrimidine (119.37 g, 777.30 mmol) in N,N-dimethylacetamide (237 mL) slowly. The flask and addition funnel were rinsed with N,N-dimethylacetamide (30 mL). A large amount of gas was evolved immediately. The mixture became a slightly cloudy orange mixture. The mixture was stirred at 0° C. for 60 min to give a light brown turbid mixture. To the mixture was slowly added [2-(trimethylsilyl)ethoxy]methyl chloride (152.40 g, 914.11 mmol) and the reaction was stirred at 0° C. for 1 h. The reaction was quenched by addition of 12 mL of H2O slowly. More water (120 mL) was added followed by methyl tert-butyl ether (MTBE) (120 mL). The mixture was stirred for 10 min. The organic layer was separated. The aqueous layer was extracted with another portion of MTBE (120 mL). The organic extracts were combined, washed with brine (120 mL×2) and concentrated under reduced pressure to give the crude product 4-chloro-7-{[2-(trimethylsilyl)ethoxy]methyl}-7H-pyrrolo[2,3-d]pyrimidine as a dark oil. Yield: 85.07 g (97%); LC-MS: 284.1 (M+H)+. It was carried to the next reaction without purification.
Step D: 4-(1H-Pyrazol-4-yl)-7-{[2-(trimethylsilyl)ethoxy]methyl}-7H-pyrrolo[2,3-d]pyrimidine
![Figure US20110224190A1-20110915-C00029]()
A 1000 mL round bottom flask was charged with 4-chloro-7-{[2-(trimethylsilyl)ethoxy]methyl}-7H-pyrrolo[2,3-d]pyrimidine (10.00 g, 35.23 mmol), 1-butanol (25.0 mL), 1-(1-ethoxyethyl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole (15.66 g, 52.85 mmol), water (25.0 mL) and potassium carbonate (12.17 g, 88.08 mmol). This solution was degased 4 times, filling with nitrogen each time. To the solution was added tetrakis(triphenylphosphine)palladium(0) (4.071 g, 3.523 mmol). The solution was degased 4 times, filling with nitrogen each time. The mixture was stirred overnight at 100° C. After being cooled to room temperature, the mixture was filtered through a bed of celite and the celite was rinsed with ethyl acetate (42 mL). The filtrate was combined, and the organic layer was separated. The aqueous layer was extracted with ethyl acetate. The organic extracts were combined and concentrated under vacuum with a bath temperature of 30-70° C. to give the final compound 4-(1H-pyrazol-4-yl)-7-{[2-(trimethylsilyl)ethoxy]methyl}-7H-pyrrolo[2,3-d]pyrimidine. Yield: 78%. LC-MS: 316.2 (M+H)+.
Step E: tert-Butyl 3-(Cyanomethyl)-3-[4-(7-{[2-(trimethylsilyl)ethoxy]methyl}-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]azetidine-1-carboxylate
![Figure US20110224190A1-20110915-C00030]()
A 2 L round bottom flask fitted with overhead stirring, septa and nitrogen inlet was charged with tert-butyl 3-(cyanomethylene)azetidine-1-carboxylate (9.17 g, 0.0472 mol), 4-(1H-pyrazol-4-yl)-7-{[2-(trimethylsilyl)ethoxy]methyl}-7H-pyrrolo[2,3-d]pyrimidine (14.9 g, 0.0472 mol) and acetonitrile (300 mL). The resulting solution was heterogeneous. To the solution was added 1,8-diazabicyclo[5.4.0]undec-7-ene (8.48 mL, 0.0567 mol) portionwise via syringe over 3 min at room temperature. The solution slowly became homogeneous and yellow in color. The reaction was allowed to stir at room temperature for 3 h. The reaction was complete by HPLC and LC/MS and was concentrated by rotary evaporation to remove acetonitrile (˜150 mL). EtOAc (100 mL) was added followed by 100 ml of 20% brine. The two phases were partitioned. The aqueous phase was extracted with 150 mL of EtOAC. The combine organic phases were dried over MgSO4, filtered and concentrated to yield an orange oil. Purification by flash chromatography (150 grams silica, 60% EtOAc/hexanes, loaded with CH2Cl2) yielded the title compound tert-butyl 3-(cyanomethyl)-3-[4-(7-{[2-(trimethylsilyl)ethoxy]methyl}-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]azetidine-1-carboxylate as a yellow oil (21.1 g, 88% yield). LC-MS: [M+H]+=510.3.
Step F: {3-[4-(7-{[2-(Trimethylsilyl)ethoxy]methyl}-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]azetidin-3-yl}acetonitrile dihydrochloride
To a solution of tert-butyl 3-(cyanomethyl)-3-[4-(7-{[2-(trimethylsilyl)ethoxy]methyl}-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]azetidine-1-carboxylate (2 g, 3.9 mmol) in 10 mL of THF was added 10 mL of 4 N HCl in dioxane. The solution was stirred at room temperature for 1 hour and concentrated in vacuo to provide 1.9 g (99%) of the title compound as a white powder solid, which was used for the next reaction without purification. LC-MS: [M+H]+=410.3.
Step G: tert-Butyl 4-{3-(Cyanomethyl)-3-[4-(7-{[2-(trimethylsilyl)ethoxy]methyl}-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]azetidin-1-yl}piperidine-1-carboxylate
![Figure US20110224190A1-20110915-C00032]()
Into the solution of {3-[4-(7-{[2-(trimethylsilyl)ethoxy]methyl}-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]azetidin-3-yl}acetonitrile dihydrochloride (2.6 g, 6.3 mmol), tert-butyl 4-oxo-1-piperidinecarboxylate (1.3 g, 6.3 mmol) in THF (30 mL) were added N,N-diisopropylethylamine (4.4 mL, 25 mmol) and sodium triacetoxyborohydride (2.2 g, 10 mmol). The mixture was stirred at room temperature overnight. After adding 20 mL of brine, the solution was extracted with EtOAc. The extract was dried over anhydrous Na2SO4 and concentrated. The residue was purified by combiflash column eluting with 30-80% EtOAc in hexanes to give the desired product, tert-butyl 4-{3-(cyanomethyl)-3-[4-(7-{[2-(trimethylsilyl)ethoxy]methyl}-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]azetidin-1-yl}piperidine-1-carboxylate. Yield: 3.2 g (86%); LC-MS: [M+H]+=593.3.
Step H: {1-Piperidin-4-yl-3-[4-(7-{[2-(trimethylsilyl)ethoxy]methyl}-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]azetidin-3-yl}acetonitrile trihydrochloride
![Figure US20110224190A1-20110915-C00033]()
To a solution of tert-butyl 4-{3-(cyanomethyl)-3-[4-(7-{[2-(trimethylsilyl)ethoxy]methyl}-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]azetidin-1-yl}piperidine-1-carboxylate (3.2 g, 5.4 mmol) in 10 mL of THF was added 10 mL of 4 N HCl in dioxane. The reaction mixture was stirred at room temperature for 2 hours. Removing solvents under reduced pressure yielded 3.25 g (100%) of {1-piperidin-4-yl-3-[4-(7-{[2-(trimethylsilyl)ethoxy]methyl}-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]azetidin-3-yl}acetonitrile trihydrochloride as a white powder solid, which was used directly in the next reaction. LC-MS: [M+H]+=493.3. 1H NMR (400 MHz, DMSO-d6): δ 9.42 (s 1H), 9.21 (s, 1H), 8.89 (s, 1H), 8.69 (s, 1H), 7.97 (s, 1H), 7.39 (d, 1H), 5.68 (s, 2H), 4.96 (d, 2H), 4.56 (m, 2H), 4.02-3.63 (m, 2H), 3.55 (s, 2H), 3.53 (t, 2H), 3.49-3.31 (3, 3H), 2.81 (m, 2H), 2.12 (d, 2H), 1.79 (m, 2H), 0.83 (t, 2H), −0.10 (s, 9H).
Step I: {1-{1-[3-Fluoro-2-(trifluoromethyl)isonicotinoyl]piperidin-4-yl}-3-[4-(7-{[2-(trimethylsilyl)ethoxy]methyl}-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]azetidin-3-yl}acetonitrile
![Figure US20110224190A1-20110915-C00034]()
A mixture of {1-piperidin-4-yl-3-[4-(7-{[2-(trimethylsilyl)ethoxy]methyl}-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]azetidin-3-yl}acetonitrile trihydrochloride (1.22 g, 2.03 mmol), 3-fluoro-2-(trifluoromethyl)isonicotinic acid (460 mg, 2.2 mmol), benzotriazol-1-yloxytris(dimethylamino)phosphonium hexafluorophosphate (1.07 g, 2.42 mmol), and triethylamine (2.0 mL, 14 mmol) in dimethylformamide (DMF) (20.0 mL) was stirred at room temperature overnight. LS-MS showed the reaction was complete. EtOAc (60 mL) and saturated NaHCO3 aqueous solution (60 mL) were added to the reaction mixture. After stirring at room temperature for 10 minutes, the organic phase was separated and the aqueous layer was extracted with EtOAc three times. The combined organic phase was washed with brine, dried over anhydrous Na2SO4, filtered and evaporated under reduced pressure. Purification by flash chromatography provided the desired product {1-{1-[3-fluoro-2-(trifluoromethyl)isonicotinoyl]piperidin-4-yl}-3-[4-(7-{[2-(trimethylsilyl)ethoxy]methyl}-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]azetidin-3-yl}acetonitrile. LC-MS: 684.3 (M+H)+.
Step J: {1-{1-[3-Fluoro-2-(trifluoromethyl)isonicotinoyl]piperidin-4-yl}-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]azetidin-3-yl}acetonitrile
![Figure US20110224190A1-20110915-C00035]()
Into a solution of {1-{1-[3-fluoro-2-(trifluoromethyl)isonicotinoyl]piperidin-4-yl}-3-[4-(7-{[2-(trimethylsilyl)ethoxy]methyl}-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]azetidin-3-yl}acetonitrile (56 mg, 0.1 mmol) in methylene chloride (1.5 mL) was added trifluoroacetic acid (1.5 mL). The mixture was stirred at room temperature for 2 hours. After removing the solvents in vacuum, the residue was dissolved in a methanol solution containing 20% ethylenediamine. After being stirred at room temperature for 1 hour, the solution was purified by HPLC (method B) to give the title compound. LC-MS: 554.3 (M+H)+; 1H NMR (400 MHz, CDCl3): 9.71 (s, 1H), 8.82 (s, 1H), 8.55 (d, J=4.6 Hz, 1H), 8.39 (s, 1H), 8.30 (s, 1H), 7.52 (t, J=4.6 Hz, 1H), 7.39 (dd, J1=3.4 Hz, J2=1.5 Hz, 1H), 6.77 (dd, J1=3.6 Hz, J2=0.7 Hz, 1H), 4.18 (m, 1H), 3.75 (m, 2H), 3.63 (dd, J1=7.8 Hz, J2=3.7 Hz, 2H), 3.45 (m, 2H), 3.38 (s, 2H), 3.11 (m, 1H), 2.57 (m, 1H), 1.72 (m, 1H), 1.60 (m, 1H), 1.48 (m, 1H), 1.40 (m, 1H).
………………………..
http://www.google.com/patents/US20130060026
Example 1Synthesis of 4-(1H-pyrazol-4-yl)-7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidine (5)
Step 1. 4-Chloro-7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidine (3)
To a flask equipped with a nitrogen inlet, an addition funnel, a thermowell, and the mechanical stirrer was added 4-chloro-7H-pyrrolo[2,3-d]pyrimidine (1, 600 g, 3.91 mol) and N,N-dimethylacetimide (DMAC, 9.6 L) at room temperature. The mixture was cooled to 0-5° C. in an ice/brine bath before solid sodium hydride (NaH, 60 wt %, 174 g, 4.35 mol, 1.1 equiv) was added in portions at 0-5° C. The reaction mixture turned into a dark solution after 15 minutes. Trimethylsilylethoxymethyl chloride (2, SEM-Cl, 763 mL, 4.31 mol, 1.1 equiv) was then added slowly via an addition funnel at a rate that the internal reaction temperature did not exceed 5° C. The reaction mixture was then stirred at 0-5° C. for 30 minutes. When the reaction was deemed complete determined by TLC and HPLC, the reaction mixture was quenched by water (1 L). The mixture was then diluted with water (12 L) and methyl tert-butyl ether (MTBE) (8 L). The two layers were separated and the aqueous layer was extracted with MTBE (8 L). The combined organic layers were washed with water (2×4 L) and brine (4 L) and solvent switched to 1-butanol. The solution of crude product (3) in 1-butanol was used in the subsequent Suzuki coupling reaction without further purification. Alternatively, the organic solution of the crude product (3) in MTBE was dried over sodium sulfate (Na2SO4). The solvents were removed under reduced pressure. The residue was then dissolved in heptane (2 L), filtered and loaded onto a silica gel (SiO2, 3.5 Kg) column eluting with heptane (6 L), 95% heptane/ethyl acetate (12 L), 90% heptane/ethyl acetate (10 L), and finally 80% heptane/ethyl acetate (10 L). The fractions containing the pure desired product were combined and concentrated under reduced pressure to give 4-chloro-7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidine (3, 987 g, 1109.8 g theoretical, 88.9% yield) as a pale yellow oil which partially solidified to an oily solid on standing at room temperature. For 3: 1H NMR (DMSO-d6, 300 MHz) δ 8.67 (s, 1H), 7.87 (d, 1H, J=3.8 Hz), 6.71 (d, 1H, J=3.6 Hz), 5.63 (s, 2H), 3.50 (t, 2H, J=7.9 Hz), 0.80 (t, 2H, J=8.1 Hz), 1.24 (s, 9H) ppm; 13C NMR (DMSO-d6, 100 MHz) δ 151.3, 150.8, 150.7, 131.5, 116.9, 99.3, 72.9, 65.8, 17.1, −1.48 ppm; C12H18ClN3OSi (MW 283.83), LCMS (EI) m/e 284/286 (M++H).
Step 2. 4-(1H-Pyrazol-4-yl)-7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidine (5)
To a reactor equipped with the overhead stirrer, a condenser, a thermowell, and a nitrogen inlet was charged water (H2O, 9.0 L), solid potassium carbonate (K2CO3, 4461 g, 32.28 mol, 2.42 equiv), 4-chloro-7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidine (3, 3597 g, 12.67 mol), 1-(1-ethoxyethyl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole (4, 3550 g, 13.34 mol, 1.05 equiv), and 1-butanol (27 L) at room temperature. The resulting reaction mixture was degassed three timed backfilling with nitrogen each time before being treated with tetrakis(triphenylphosphine)palladium(0) (Pd(PPh3)4, 46 g, 0.040 mol, 0.003 equiv) at room temperature. The resulting reaction mixture was heated to gentle reflux (about 90° C.) for 1-4 hours. When the reaction was deemed complete determined by HPLC, the reaction mixture was gradually cooled down to room temperature before being filtered through a Celite bed. The Celite bed was washed with ethyl acetate (2×2 L) before the filtrates and washing solution were combined. The two layers were separated, and the aqueous layer was extracted with ethyl acetate (12 L). The combined organic layers were concentrated under reduced pressure to remove solvents, and the crude 4-(1-(1-ethoxyethyl)-1H-pyrazol-4-yl)-7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidine (6) was directly charged back to the reactor with tetrahydrofuran (THF, 4.2 L) for the subsequent acid-promoted de-protection reaction without further purification.
To a suspension of crude 4-(1-(1-ethoxyethyl)-1H-pyrazol-4-yl)-7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidine (6), made as described above, in tetrahydrofuran (THF, 4.2 L) in the reactor was charged water (H2O, 20.8 L), and a 10% aqueous HCl solution (16.2 L, 45.89 mol, 3.44 equiv) at room temperature. The resulting reaction mixture was stirred at 16-30° C. for 2-5 hours. When the reaction was deemed complete by HPLC analysis, the reaction mixture was treated with a 30% aqueous sodium hydroxide (NaOH) solution (4 L, 50.42 mol, 3.78 equiv) at room temperature. The resulting reaction mixture was stirred at room temperature for 1-2 hours. The solids were collected by filtration and washed with water (2×5 L). The wet cake was charged back to the reactor with acetonitrile (21.6 L), and resulting suspension was heated to gentle reflux for 1-2 hours. The clear solution was then gradually cooled down to room temperature with stirring, and solids were precipitated out from the solution with cooling. The mixture was stirred at room temperature for an additional 1-2 hours. The solids were collected by filtration, washed with acetonitrile (2×3.5 L), and dried in oven under reduced pressure at 45-55° C. to constant weight to afford 4-(1H-pyrazol-4-yl)-7-(2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidine (5, 3281.7 g, 3996.8 g theoretical, 82.1% yield) as white crystalline solids (99.5 area % by HPLC). For 5: 1H NMR (DMSO-d6, 400 MHz) δ 13.41 (br. s, 1H), 8.74 (s, 1H), 8.67 (br. s, 1H), 8.35 (br. s, 1H), 7.72 (d, 1H, J=3.7 Hz), 7.10 (d, 1H, J=3.7 Hz), 5.61 (s, 2H), 3.51 (t, 2H, J=8.2 Hz), 0.81 (t, 2H, J=8.2 Hz), 0.13 (s, 9H) ppm; C15H21N5OSi (MW, 315.45), LCMS (EI) m/e 316 (M++H).
Example 2tert-Butyl 3-(cyanomethylene)azetidine-1-carboxylate (13)
Step 1. 1-Benzhydrylazetidin-3-ol hydrochloride (9)
A solution of diphenylmethanamine (7, 2737 g, 15.0 mol, 1.04 equiv) in methanol (MeOH, 6 L) was treated with 2-(chloromethyl)oxirane (8, 1330 g, 14.5 mol) from an addition funnel at room temperature. During the initial addition a slight endotherm was noticed. The resulting reaction mixture was stirred at room temperature for 3 days before being warmed to reflux for an additional 3 days. When TLC showed that the reaction was deemed complete, the reaction mixture was first cooled down to room temperature and then to 0-5° C. in an ice bath. The solids were collected by filtration and washed with acetone (4 L) to give the first crop of the crude desired product (9, 1516 g). The filtrate was concentrated under reduced pressure and the resulting semisolid was diluted with acetone (1 L). This solid was then collected by filtration to give the second crop of the crude desired product (9, 221 g). The crude product, 1-benzhydrylazetidin-3-ol hydrochloride (9, 1737 g, 3998.7 g theoretical, 43.4% yield), was found to be sufficiently pure to be used in the subsequent reaction without further purification. For 9: 1H NMR (DMSO-d6, 300 MHz), δ 12.28 (br. d, 1H), 7.7 (m, 5H), 7.49 (m, 5H), 6.38 (d, 1H), 4.72 (br. s, 1H), 4.46 (m, 1H), 4.12 (m, 2H), 3.85 (m, 2H) ppm; C16H18ClNO (free base of 9, C16K7NO MW, 239.31), LCMS (EI) m/e 240 (M++H).
Step 2. tert-Butyl 3-hydroxyazetidine-1-carboxylate (10)
A suspension of 1-benzhydrylazetidin-3-ol hydrochloride (9, 625 g, 2.27 mol) in a 10% solution of aqueous sodium carbonate (Na2CO3, 5 L) and dichloromethane (CH2Cl2, 5 L) was stirred at room temperature until all solids were dissolved. The two layers were separated, and the aqueous layer was extracted with dichloromethane (CH2Cl2, 2 L). The combined organics extracts were dried over sodium sulfate (Na2SO4) and concentrated under reduced pressure. This resulting crude free base of 9 was then dissolved in THF (6 L) and the solution was placed into a large Parr bomb. Di-tert-butyl dicarbonate (BOC2O, 545 g, 2.5 mol, 1.1 equiv) and 20% palladium (Pd) on carbon (125 g, 50% wet) were added to the Parr bomb. The vessel was charged to 30 psi with hydrogen gas (H2) and stirred under steady hydrogen atmosphere (vessel was recharged three times to maintain the pressure at 30 psi) at room temperature for 18 h. When HPLC showed that the reaction was complete (when no more hydrogen was taken up), the reaction mixture was filtered through a Celite pad and the Celite pad was washed with THF (4 L). The filtrates were concentrated under reduced pressure to remove the solvent and the residue was loaded onto a Biotage 150 column with a minimum amount of dichloromethane (CH2Cl2). The column was eluted with 20-50% ethyl acetate in heptane and the fractions containing the pure desired product (10) were collected and combined. The solvents were removed under reduced pressure to afford tert-butyl 3-hydroxyazetidine-1-carboxylate (10, 357 g, 393.2 g theoretical, 90.8% yield) as colorless oil, which solidified upon standing at room temperature in vacuum. For 10: 1HNMR (CDCl3, 300 MHz), δ 4.56 (m 1H), 4.13 (m, 2H), 3.81 (m, 2H), 1.43 (s, 9H) ppm.
Step 3. tert-Butyl 3-oxoazetidine-1-carboxylate (11)
A solution of tert-butyl 3-hydroxyazetidine-1-carboxylate (10, 50 g, 289 mmol) in ethyl acetate (400 mL) was cooled to 0° C. The resulting solution was then treated with solid TEMPO (0.5 g, 3.2 mmol, 0.011 equiv) and a solution of potassium bromide (KBr, 3.9 g, 33.2 mmol, 0.115 equiv) in water (60 mL) at 0-5° C. While keeping the reaction temperature between 0-5° C. a solution of saturated aqueous sodium bicarbonate (NaHCO3, 450 mL) and an aqueous sodium hypochlorite solution (NaClO, 10-13% available chlorine, 450 mL) were added. Once the solution of sodium hypochlorite was added, the color of the reaction mixture was changed immediately. When additional amount of sodium hypochlorite solution was added, the color of the reaction mixture was gradually faded. When TLC showed that all of the starting material was consumed, the color of the reaction mixture was no longer changed. The reaction mixture was then diluted with ethyl acetate (EtOAc, 500 mL) and two layers were separated. The organic layer was washed with water (500 mL) and the saturated aqueous sodium chloride solution (500 mL) and dried over sodium sulfate (Na2SO4). The solvent was then removed under reduced pressure to give the crude product, tert-butyl 3-oxoazetidine-1-carboxylate (11, 48 g, 49.47 g theoretical, 97% yield), which was found to be sufficiently pure and was used directly in the subsequent reaction without further purification. For crude 11: 1HNMR (CDCl3, 300 MHz), δ 4.65 (s, 4H), 1.42 (s, 9H) ppm.
Step 4. tert-Butyl 3-(cyanomethylene)azetidine-1-carboxylate (13)
Diethyl cyanomethyl phosphate (12, 745 g, 4.20 mol, 1.20 equiv) and anhydrous tetrahydrofuran (THF, 9 L) was added to a four-neck flask equipped with a thermowell, an addition funnel and the nitrogen protection tube at room temperature. The solution was cooled with an ice-methanol bath to −14° C. and a 1.0 M solution of potassium tert-butoxide (t-BuOK) in anhydrous tetrahydrofuran (THF, 3.85 L, 3.85 mol, 1.1 equiv) was added over 20 minutes keeping the reaction temperature below −5° C. The resulting reaction mixture was stirred for 3 hours at −10° C. and a solution of 1-tert-butoxycarbonyl-3-azetidinone (11, 600 g, 3.50 mol) in anhydrous tetrahydrofuran (THF, 2 L) was added over 2 h keeping the internal temperature below −5° C. The reaction mixture was stirred at −5 to −10° C. over 1 hour and then slowly warmed up to room temperature and stirred at room temperature for overnight. The reaction mixture was then diluted with water (4.5 L) and saturated aqueous sodium chloride solution (NaCl, 4.5 L) and extracted with ethyl acetate (EtOAc, 2×9 L). The combined organic layers were washed with brine (6 L) and dried over anhydrous sodium sulfate (Na2SO4). The organic solvent was removed under reduced pressure and the residue was diluted with dichloromethane (CH2Cl2, 4 L) before being absorbed onto silica gel (SiO2, 1.5 Kg). The crude product, which was absorbed on silica gel, was purified by flash column chromatography (SiO2, 3.5 Kg, 0-25% EtOAc/hexanes gradient elution) to afford tert-butyl 3-(cyanomethylene)azetidine-1-carboxylate (13, 414.7 g, 679.8 g theoretical, 61% yield) as white solid. For 13: 1H NMR (CDCl3, 300 MHz), δ 5.40 (m, 1H), 4.70 (m, 2H), 4.61 (m, 2H), 1.46 (s, 9H) ppm; C10H14N2O2 (MW, 194.23), LCMS (EI) m/e 217 (M′+Na).
Example 3(3-Fluoro-2-(trifluoromethyl)pyridin-4-yl)(1,4-dioxa-8-azaspiro[4,5]decan-8-yl)methanone (17)
Step 1. 1,4-Dioxa-8-azaspiro[4.5]decane (15)
To a 30 L reactor equipped with a mechanic stirrer, an addition funnel and a septum was charged sodium hydroxide (NaOH, 1.4 kg, 35 mol) and water (7 L, 3.13 kg, 17.43 mol). To the solution thus obtained was added 1,4-dioxa-8-azaspiro[4.5]decane hydrochloric acid (14, 3.13 kg, 17.43 mol). The mixture was stirred at 25° C. for 30 minutes. Then the solution was saturated with sodium chloride (1.3 kg) and extracted with 2-methyl-tetrahydrofuran (3×7 L). The combined organic layer was dried with anhydrous sodium sulfate (1.3 kg), filtered and concentrated under reduced pressure (70 mmHg) at 50° C. The yellow oil thus obtained was distilled under reduced pressure (80 mmHg, bp: 115° C. to 120° C.) to give compound 15 (2.34 kg, 16.36 mol, 93.8%) as a clear oil, which was used directly in the subsequent coupling reaction.
Step 2. (3-Fluoro-2-(trifluoromethyl)pyridin-4-yl)(1,4-dioxa-8-azaspiro[4,5]decan-8-yl)methanone (17)
To a dried 100 L reactor equipped with a mechanic stirrer, an addition funnel, a thermometer and a vacuum outlet were placed 3-fluoro-2-(trifluoromethyl)isonicotinic acid (16, 3.0 kg, 14.35 mol), benzotriazol-1-yloxytris(dimethylamino)phosphonium hexafluorophosphate (BOP reagent, 7.6 kg, 17.2 mol, 1.20 equiv) in dimethylformamide (DMF, 18 L). To the resulting solution was added 1,4-dioxa-8-azaspiro[4.5]decane (15, 2.34 kg, 16.36 mol, 1.14 equiv) with stirring over 20 minutes. Triethylamine (Et3N, 4 L, 28.67 mol, 2.00 equiv) was then added over 1 hour. The temperature was kept between 5° C. and 10° C. during the additions. The dark brown solution thus obtained was stirred for 12 hours at 20° C. and then chilled to 10° C. With vigorous stirring, 18 L of saturated sodium bicarbonate solution and 36 L of water were sequentially added and the temperature was kept under 15° C. The precipitation (filter cake) thus obtained was collected by filtration. The aqueous phase was then saturated with 12 kg of solid sodium chloride and extracted with EtOAc (2×18 L). The combined organic layer was washed with saturated sodium bicarbonate solution (18 L), and water (2×18 L) in sequence. The filter cake from the previous filtration was dissolved back in the organic phase. The dark brown solution thus obtained was washed twice with 18 L of water each and then concentrated under reduced pressure (40-50° C., 30 mm Hg) to give 5.0 kg of the crude product as viscous brown oil. The crude product 17 obtained above was dissolved in EtOH (8.15 L) at 50° C. Water (16.3 L) was added over 30 minutes. The brown solution was seeded, cooled to 20° C. over 3 hours with stirring and stirred at 20° C. for 12 h. The precipitate formed was filtered, washed with a mixture of EtOH and water (EtOH:H2O=1:20, 2 L) and dried under reduced pressure (50 mmHg) at 60° C. for 24 hours to afford (3-fluoro-2-(trifluoromethyl)pyridin-4-yl)(1,4-dioxa-8-azaspiro[4,5]decan-8-yl)methanone (17, 3.98 kg, 11.92 mol, 83.1%) as a white powder. For 17: 1H NMR (300 MHz, (CD3)2SO) δ 8.64 (d, 3JHH=4.68 Hz, 1H, NCH in pyridine), 7.92 (dd, 3JHH=4.68 Hz, 4JHF=4.68 Hz, 1H, NCCH in pyridine), 3.87-3.91 (m, 4H, OCH2CH2O), 3.70 (br s, 2H, one of NCH2 in piperidine rine, one of another NCH2 in piperidine ring, both in axial position), 3.26 (t, 3JHH=5.86 Hz, 2H, one of NCH2 in piperidine rine, one of another NCH2 in piperidine ring, both in equatorial position), 1.67 (d, 3JHH=5.86 Hz, 2H, one of NCCH2 in piperidine ring, one of another NCCH2 in piperidine ring, both in equatorial position), 1.58 (br s, 2H, one of NCCH2 in piperidine ring, one of another NCCH2 in piperidine ring, both in axial position) ppm; 13C NMR (75 MHz, (CD3)2SO) δ 161.03 (N—C═O), 151.16 (d, 1JCF=266.03 Hz, C—F), 146.85 (d, 4JCF=4.32 Hz, NCH in pyridine), 135.24 (d, 2JCF=11.51 Hz, C—C═O), 135.02 (quartet, 2JCF=34.57 Hz, NCCF3), 128.24 (d, 4JCF=7.48 Hz, NCCH in pyridine), 119.43 (d×quartet, 1JCF=274.38 Hz, 3JCF=4.89 Hz, CF3), 106.74 (OCO), 64.60 (OCCO), 45.34 (NC in piperidine ring), 39.62 (NC in piperidine ring), 34.79 (NCC in piperidine ring), 34.10 (NCC in piperidine ring) ppm; 19F NMR (282 MHz, (CD3)2SO) δ-64.69 (d, 4JFF=15.85 Hz, F3C), −129.26 (d×quartet, 4JFF=15.85 Hz, 4JFH=3.96 Hz, FC) ppm; C14H14F4N2O3 (MW, 334.27), LCMS (EI) m/e 335.1 (M++H).
Example 4(3-Fluoro-2-(trifluoromethyl)pyridin-4-yl) (1,4-dioxa-8-azaspiro[4,5]decan-8-yl)methanone (18)
![Figure US20130060026A1-20130307-C00022]()
In a 5 L 4-necked round bottom flask equipped with a mechanical stirrer, a thermocouple, an addition funnel and a nitrogen inlet was placed (3-fluoro-2-(trifluoromethyl)pyridin-4-yl)(1,4-dioxa-8-azaspiro[4,5]decan-8-yl)methanone (17, 100 g, 0.299 mol) in acetonitrile (ACN, 400 mL) at room temperature. The resultant solution was cooled to below 10° C. To the reaction mixture was added 6.0 N aqueous hydrochloric acid (HCl, 450 mL, 2.70 mol, 9.0 equiv), while the internal temperature was kept below 10° C. The resulting reaction mixture was then warmed to room temperature and an additional amount of 6.0 N aqueous hydrochloric acid (HCl, 1050 mL, 6.30 mol, 21.0 equiv) was slowly introduced to the reaction mixture at room temperature in 8 hours via the addition funnel. The reaction mixture was then cooled to 0° C. before being treated with 30% aqueous sodium hydroxide (NaOH, 860 mL, 8.57 mmol, 28.6 equiv) while the internal temperature was kept at below 10° C. The resulting reaction mixture was subsequently warmed to room temperature prior to addition of solid sodium bicarbonate (NaHCO3, 85.0 g, 1.01 mol, 3.37 equiv) in 1 hour. The mixture was then extracted with EtOAc (2×1.2 L), and the combined organic phase was washed with 16% aqueous sodium chloride solution (2×800 mL) and concentrated to approximately 1.0 L by vacuum distillation. Heptane (2.1 L) was added to the residue, and the resulting mixture was concentrated to 1.0 L by vacuum distillation. To the concentrated mixture was added heptane (2.1 L). The resulting white slurry was then concentrated to 1.0 L by vacuum distillation. To the white slurry was then added methyl tert-butyl ether (MTBE, 1.94 L). The white turbid was heated to 40° C. to obtain a clear solution. The resulting solution was concentrated to about 1.0 L by vacuum distillation. The mixture was stirred at room temperature for 1 hour. The white precipitate was collected by filtration with pulling vacuum. The filter cake was washed with heptane (400 mL) and dried on the filter under nitrogen with pulling vacuum to provide compound 18 (78.3 g, 90.1%) as an off-white solid. For 18: 1H NMR (300 MHz, (CD3)2SO) δ 8.68 (d, 3JHH=4.69 Hz, 1H, NCH in pyridine), 7.97 (dd, 3JHH=4.69 Hz, 4JHF=4.69 Hz, 1H, NCCH in pyridine), 3.92 (br s, 2H, one of NCH2 in piperidine rine, one of another NCH2 in piperidine ring, both in axial position), 3.54 (t, 3JHH=6.15 Hz, 2H, one of NCH2 in piperidine rine, one of another NCH2 in piperidine ring, both in equatorial position), 2.48 (t, 3JHH=6.44 Hz, 2H, NCCH2), 2.34 (t, 3JHE=6.15 Hz, 2H, NCCH2) ppm; 13C NMR (75 MHz, (CD3)2SO) δ 207.17 (C═O), 161.66 (N—C═O), 151.26 (d, 1JCF=266.89 Hz, C—F), 146.90 (d, 4JCF=6.05 Hz, NCH in pyridine), 135.56 (C—C═O), 134.78-135.56 (m, NCCF3), 128.27 (d, 3JCF=7.19 Hz, NCCH in pyridine), 119.52 (d×quartet, 1JCF=274.38 Hz, 3JCF=4.89 Hz, CF3), 45.10 (NC in piperidine ring) ppm, one carbon (NCC in piperidine ring) missing due to overlap with (CD3)2SO; 19F NMR (282 MHz, (CD3)2SO) δ-64.58 (d, 4JFF=15.85 Hz, F3C), −128.90 (d×quartet, 4JFF=15.85 Hz, 4JFH=4.05 Hz, FC) ppm; C12H10F4N2O2 (MW, 290.21), LCMS (EI) m/e 291.1 (M++H).
Example 53-[4-(7-{[2-(Trimethylsilyl)ethoxy]methyl}-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]azetidin-3-yl}acetonitrile dihydrochloride (20)
Step 1. tent-Butyl 3-(cyanomethyl)-3-(4-(7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)azetidine-1-carboxylate (19)
In a dried 30 L reactor equipped with a mechanic stirrer, a thermometer, an addition funnel and a vacuum outlet were placed 4-(1H-pyrazol-4-yl)-7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidine (5, 4.50 kg, 14.28 mol), tert-butyl 3-(cyanomethylene)azetidine-1-carboxylate (13, 3.12 kg, 16.08 mol, 1.126 equiv) in acetonitrile (9 L) at 20±5° C. To the resultant pink suspension was added 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU, 225 mL, 1.48 mol, 0.10 equiv) over 40 minutes. The batch temperature was kept between 10° C. and 20° C. during addition. The brown solution obtained was stirred at 20° C. for 3 hours. After the reaction was complete, water (18 L) was added with stirring over 80 minutes at 20° C. The mixture was seeded and the seeded mixture was stirred at room temperature for 12 hours. The solids were collected by filtration and the filter cake was washed with a mixture of acetonitrile and water (1:2, 9 L) and dried in a vacuum oven with nitrogen purge for 12 hours at 60° C. to provide the crude product (19, 7.34 kg) as a light yellow powder. The crude product obtained above was dissolved in methyl tert-butyl ether (MTBE, 22 L) at 60° C. in a 50 L reactor equipped with a mechanic stirrer, a thermometer, an addition funnel and a septum. Hexanes (22 L) was added over 1 hour at 60° C. The solution was then seeded, cooled to 20° C. over 3 hours and stirred at 20° C. for 12 hours. The precipitation was collected by filtration. The resultant cake was washed with a mixture of MTBE and hexane (1:15, 3 L) and dried in a vacuum oven for 10 hours at 50° C. to provide the compound 19 (6.83 kg, 13.42 mol, 94.0%) as a white powder. For 19: 1H NMR (400 MHz, CDCl3) δ 8.87 (s, 1H), 8.46 (d, J=0.6 Hz, 1H), 8.36 (d, J=0.7 Hz, 1H), 7.44 (d, J=3.7 Hz, 1H), 6.82 (d, J=3.7 Hz, 1H), 5.69 (s, 2H), 4.57 (d, J=9.6 Hz, 2H), 4.32 (d, J=9.5 Hz, 2H), 3.59-3.49 (m, 2H), 3.35 (s, 2H), 1.49 (s, 9H), 0.96-0.87 (m, 2H), −0.03-−0.10 (s, 9H) ppm; 13C NMR (101 MHz, CDCl3) δ 157.22, 153.67, 153.24, 151.62, 142.13, 130.16, 129.67, 124.47, 116.72, 115.79, 102.12, 82.54, 74.23, 68.01, 60.25, 58.23, 29.65, 29.52, 19.15, −0.26 ppm; C25H35N7O3Si (MW, 509.68), LCMS (EI) m/e 510.1 (M++H).
Step 2. 3-[4-(7-{[2-(Trimethylsilyl)ethoxy]methyl}-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]azetidin-3-yl}acetonitrile dihydrochloride (20)
In a 2 L 4-necked round bottom flask equipped with a mechanical stirrer, a thermocouple, an addition funnel and a nitrogen inlet was added compound 19 (55.0 g, 0.108 mol) and methanol (MeOH, 440 mL) at 20±5° C. The resulting white turbid was stirred for 20 minutes at room temperature to provide a light yellow solution. A solution of hydrochloric acid (HCl) in isopropanol (5.25 M, 165 mL, 0.866 mol, 8.02 equiv) was then added to the reaction mixture via the addition funnel in 5 minutes. The resulting reaction mixture was then heated to 40° C. by a heating mantle. After 2 hours at 40° C., water (165 mL, 9.17 mol, 84.8 equiv) was added to the reaction mixture via the addition funnel to provide a light green solution at 40° C. Methyl tert-butyl ether (MTBE, 440 mL) was added to the resulting mixture via the addition funnel at 40° C. The resulting mixture was slowly cooled to 10° C. The solids were collected by filtration and washed with MTBE (2×220 mL). The white solids were dried in the filter under nitrogen with a pulling vacuum for 18 hours to afford compound 20 (52.2 g, KF water content 5.42%, yield 94.9%). For 20: 1H NMR (400 MHz, (CD3)2SO) δ 10.39 (brs, 1H), 10.16 (brs, 1H), 9.61 (s, 1H), 9.12 (s, 1H), 9.02 (s, 1H), 8.27-8.21 (d, J=3.8 Hz, 1H), 7.72-7.66 (d, J=3.8 Hz, 1H), 5.82 (s, 2H), 4.88-4.77 (m, 2H), 4.53-4.44 (m, 2H), 4.12 (s, 2H), 3.69-3.60 (m, 2H), 0.98-0.89 (m, 2H), 0.01 (s, 9H) ppm; 13C NMR (101 MHz, (CD3)2SO) δ 151.25, 146.45, 145.09, 140.75, 133.38, 132.44, 116.20, 116.09, 112.79, 102.88, 73.07, 66.14, 59.16, 53.69, 26.44, 17.15, −1.36 ppm; C20H29Cl2N7OSi (free base of 20, C20H27N7OSi, MW 409.56), LCMS (EI) m/e 410.2 (M++H).
Example 62-(1-(1-(3-Fluoro-2-(trifluoromethyl)isonicotinoyl)piperidin-4-yl)-3-(4-(7-(2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)azetidin-3-yl)acetonitrile (21)
![Figure US20130060026A1-20130307-C00024]()
In a 100 L dried reactor equipped with a mechanical stirrer, a thermocouple, a condenser, and a nitrogen inlet was added (20, 3.24 kg, 6.715 mol) and dichloromethane (32 L) at 20±5° C. The mixture was stirred at room temperature for 10 minutes before being treated with triethylamine (TEA, 1.36 kg, 13.44 mol, 2.00 equiv) at an addition rate which keeping the internal temperature at 15-30° C. Compound 18 (2.01 kg, 6.926 mol, 1.03 equiv) was then added to the reactor at room temperature. After 10 minutes, sodium triacetoxyborohydride (NaBH(OAc)3, 2.28 kg, 10.75 mol, 1.60 equiv) was added portion wise to the reactor in 1 hour while the internal temperature was kept at 15-30° C. The resulting reaction mixture was stirred at 15-30° C. for an additional one hour. Once the reductive amination reaction is deemed complete, the reaction mixture was treated with a 4% aqueous sodium bicarbonate solution (NaHCO3, 32 L) to adjust the pH to 7-8. After stirring for 30 minutes at room temperature, the two phases were separated. The aqueous phase was extracted with dichloromethane (29 L). The combined organic phase was sequentially washed with 0.1 N aqueous hydrochloric acid solution (16 L), 4% aqueous sodium bicarbonate solution (16 L), 8% aqueous sodium chloride solution (2×16 L). The resultant organic phase was partially concentrated and filtered. The filtrate was subjected to solvent exchange by gradually adding acetonitrile (65 L) under vacuum. The white solids were collected by filtration, washed with acetonitrile (10 L) and dried at 40-50° C. in a vacuum oven with nitrogen purge to afford compound 21 (4.26 kg, 6.23 mol, 92.9%). For 21: 1H NMR (500 MHz, (CD3)2SO) δ 8.84 (s, 1H), 8.76 (s, 1H), 8.66 (d, J=4.7 Hz, 1H), 8.43 (s, 1H), 7.90 (t, J=4.7 Hz, 1H), 7.78 (d, J=3.7 Hz, 1H), 7.17 (d, J=3.7 Hz, 1H), 5.63 (s, 2H), 4.07 (dt, J=11.1, 4.9 Hz, 1H), 3.75 (d, J=7.8 Hz, 2H), 3.57 (dd, J=10.2, 7.8 Hz, 2H), 3.55 (s, 2h), 3.52 (dd, J=8.5, 7.4 Hz, 2H), 3.41 (dq, J=13.3, 4.3 Hz, 1H), 3.26 (t, J=10.0 Hz, 1H), 3.07 (ddd, J=13.1, 9.4, 3.2 Hz, 1H), 2.56 (dt, J=8.5, 4.7 Hz, 1H), 1.81-1.73 (m, 1H), 1.63 (m, 1H), 1.29 (m, 1H), 1.21 (m, 1H), 0.82 (dd, J=8.5, 7.4 Hz, 2H), −0.12 (s, 9H) ppm; 13C NMR (101 MHz, (CD3)2SO) δ 161.68, (154.91, 152.27), 153.08, 152.69, 151.53, 147.69, 140.96, (136.19, 136.02), (136.48, 136.36, 136.13, 136.0, 135.78, 135.66, 135.43, 135.32), 131.43, 130.84, 129.03, (126.17, 123.42, 120.69), 117.99, 122.77, 118.78, 114.71, 102.02, 73.73, 67.04, 62.86, 61.88, 58.51, 45.63, 30.03, 29.30, 28.60, 18.52, 0.00 ppm; C32H37F4N9O2Si (MW, 683.77), LCMS (EI) m/e 684.2 (M++H).
Example 72-(3-(4-(7H-Pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-1-(1-(3-fluoro-2-(trifluoromethyl)isonicotinoyl)piperidin-4-yl)azetidin-3-yl)acetonitrile (22)
![Figure US20130060026A1-20130307-C00025]()
BASE OF INCB 39110
To a 250 mL 4-necked round bottom flask equipped with a mechanical stirrer, a thermocouple, an addition funnel and a nitrogen inlet was added compound 21 (9.25 g, 13.52 mmol, KF water content 3.50%) and acetonitrile (74 mL) at 20±5° C. The resulting white slurry was cooled to below 5° C. Boron trifluoride diethyl etherate (BF3.OEt2, 6.46 mL, 51.37 mmol, 3.80 equiv) was then added at a rate while the internal temperature was kept at below 5.0° C. The reaction mixture was then warmed to 20±5° C. After stirring at 20±5° C. for 18 hours, the reaction mixture was cooled to 0-5° C. and an additional amount of BF3.OEt2 (0.34 mL, 2.70 mmol, 0.2 equiv) was introduced to the reaction mixture at below 5.0° C. The resulting reaction mixture was warmed to 20±5° C., and kept stirring at room temperature for an additional 5 hours. The reaction mixture was then cooled to 0-5° C. before water (12.17 mL, 0.676 mol, 50 equiv) was added. The internal temperature was kept at below 5.0° C. during addition of water. The resultant mixture was warmed to 20±5° C. and kept stirring at room temperature for 2 hours. The reaction mixture was then cooled to 0-5° C. and aqueous ammonium hydroxide (NH4OH, 5 N, 121.7 mmol, 9.0 equiv) was added. During addition of aqueous ammonium hydroxide solution, the internal temperature was kept at below 5.0° C. The resulting reaction mixture was warmed to 20±5° C. and stirred at room temperature for 20 hours. Once the SEM-deprotection was deemed complete, the reaction mixture was filtered, and the solids were washed with EtOAc (9.25 mL). The filtrates were combined and diluted with EtOAc (74 mL). The diluted organic solution was washed with 13% aqueous sodium chloride solution (46.2 mL). The organic phase was then diluted with EtOAc (55.5 mL) before being concentrated to a minimum volume under reduced pressure. EtOAc (120 mL) was added to the residue, and the resulting solution was stirred at 20±5° C. for 30 minutes. The solution was then washed with 7% aqueous sodium bicarbonate solution (2×46 mL) and 13% aqueous sodium bicarbonate solution (46 mL). The resultant organic phase was diluted with EtOAc (46 mL) and treated with water (64 mL) at 50±5° C. for 30 minutes. The mixture was cooled to 20±5° C. and the two phases were separated. The organic phase was treated with water (64 mL) at 50±5° C. for 30 minutes for the second time. The mixture was cooled to 20±5° C. and the two phases were separated. The resultant organic phase was concentrated to afford crude compound 22 (free base), which was further purified by column chromatography (SiO2, 330 g, gradient elution with 0-10% of MeOH in EtOAc) to afford analytically pure free base (22, 7.00 g, 93.5%) as an off-white solid. For 22:
1H NMR (400 MHz, (CD3)2SO) δ 12.17 (d, J=2.8 Hz, 1H), 8.85 (s, 1H), 8.70 (m, 2H), 8.45 (s, 1H), 7.93 (t, J=4.7 Hz, 1H), 7.63 (dd, J=3.6, 2.3 Hz, 1H), 7.09 (dd, J=3.6, 1.7 Hz, 1H), 4.10 (m, 1H), 3.78 (d, J=7.9 Hz, 2H), 3.61 (t, J=7.9 Hz, 1H), 3.58 (s, 2H), 3.46 (m, 1H), 3.28 (t, J=10.5 Hz, 1H), 3.09 (ddd, J=13.2, 9.5, 3.1 Hz, 1H), 2.58 (m, 1H), 1.83-1.75 (m, 1H), 1.70-1.63 (m, 1H), 1.35-1.21 (m, 2H) ppm;
13C NMR (101 MHz, (CD3)2SO) δ 160.28, (153.51, 150.86), 152.20, 150.94, 149.62, (146.30, 146.25), 139.48, (134.78, 134.61), (135.04, 134.92, 134.72, 134.60, 134.38, 134.26, 134.03, 133.92), 129.22, 127.62, 126.84, 121.99, 122.04, (124.77, 122.02, 119.19, 116.52), 117.39, 113.00, 99.99, 61.47, 60.49, 57.05, 44.23, 28.62, 27.88, 27.19 ppm;
C26H23F4N9O (MW, 553.51), LCMS (EI) m/e 554.1 (M′+H).
ADIPATE
Example 8
2-(3-(4-(7H-Pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-1-(1-(3-fluoro-2-(trifluoromethyl)isonicotinoyl)piperidin-4-yl)azetidin-3-yl)acetonitrile adipate (25)
Step 1. 2-(3-(4-(7H-Pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-1-(1-(3-fluoro-2-(trifluoromethyl)isonicotinoyl)piperidin-4-yl)azetidin-3-yl)acetonitrile adipate crude salt (24)
The process of making compound 22 in Example 7 was followed, except that the final organic phase was concentrated by vacuum distillation to the minimum volume to afford crude compound 22, which was not isolated but was directly used in subsequent adipate salt formation process. To the concentrated residue which containing crude compound 22 was added methanol (200 mL) at room temperature. The mixture was the concentrated by vacuum distillation to a minimum volume. The residue was then added methanol (75 mL) and the resulting solution was heated to reflux for 2 hours. Methyl isobutyl ketone (MIBK, 75 mL) was added to the solution and the resulting mixture was distilled under vacuum to about 30 mL while the internal temperature was kept at 40-50° C. Methanol (75 mL) was added and the resulting mixture was heated to reflux for 2 hours. To the solution was added MIBK (75 mL). The mixture was distilled again under vacuum to about 30 mL while the internal temperature was kept at 40-50° C. To the solution was added a solution of adipic acid (23, 2.15 g, 14.77 mmol) in methanol (75 mL). The resultant solution was then heated to reflux for 2 hours. MIBK (75 mL) was added. The mixture was distilled under vacuum to about 60 mL while the internal temperature was kept at 40-50° C. Heating was stopped and heptane (52.5 mL) was added over 1-2 hours. The resultant mixture was stirred at 20±5° C. for 3-4 hours. The white precipitates were collected by filtration, and the filter cake was washed with heptane (2×15 mL). The solid was dried on the filter under nitrogen with a pulling vacuum at 20±5° C. for 12 hours to provide compound 24 (crude adipate salt, 8.98 g, 12.84 mmol., 95.0%). For 24: 1H NMR (400 MHz, (CD3)2SO) δ 12.16 (s, 1H), 12.05 (brs, 2H), 8.85 (s, 1H), 8.72 (s, 1H), 8.69 (d, J=4.7 Hz, 1H), 8.45 (s, 1H), 7.93 (t, J=4.7 Hz, 1H), 7.63 (dd, J=3.6, 2.3 Hz, 1H), 7.09 (dd, J=3.6, 1.7 Hz, 1H), δ 4.11 (dt, J=11.0, 4.4 Hz, 1H), 3.77 (d, J=7.8 Hz, 2H), 3.60 (t, J=7.8 Hz, 2H), 3.58 (s, 2H), 3.44 (dt, J=14.4, 4.6 Hz, 1H), 3.28 (t, J=10.4 Hz, 1H), 3.09 (ddd, J=13.2, 9.6, 3.2 Hz, 1H), 2.58 (tt, J=8.6, 3.5 Hz, 1H), 2.28-2.17 (m, 4H), 1.83-1.74 (m, 1H), 1.67 (d, J=11.0 Hz, 1H), 1.59-1.46 (m, 4H), 1.37-1.21 (m, 2H) ppm; 13C NMR (101 MHz, (CD3)2SO) δ 174.38, 160.29, (153.52, 150.87), 152.20, 150.94, 149.63, (146.30, 146.25), 139.48, (134.79, 134.62), (135.08, 134.97, 134.74, 134.62, 134.38, 134.28, 134.04, 133.93), 129.21, 127.62, 126.84, 122.05, (124.75, 122.02, 119.29, 116.54), 117.39, 113.01, 99.99, 61.47, 60.50, 57.06, 44.24, 33.42, 30.70, 28.63, 27.89, 27.20, 24.07 ppm; C32H33F4N9O5 (Mol. Wt: 699.66; 24: C26H23F4N9O, MW 553.51), LCMS (EI) m/e 554.0 (M++H).
Step 2.
2-(3-(4-(7H-Pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-1-(1-(3-fluoro-2-(trifluoromethyl)isonicotinoyl)piperidin-4-yl)azetidin-3-yl)acetonitrile adipate (25)
In a 100 L dried reactor equipped with a mechanical stirrer, a thermocouple, an addition funnel and a nitrogen inlet was added compound 24 (3.40 kg, 4.86 mol) and acetone (23.8 L). The resulting white turbid was heated to 55-60° C. to provide a clear solution. The resultant solution was filtered through an in-line filter to another 100 L reactor. Heptane (23.8 L) was filtered through an in-line filter to a separated 50 L reactor. The filtered heptane was then charged to the acetone solution in the 100 L reactor at a rate while the internal temperature was kept at 55-60° C. The reaction mixture in the 100 L reactor was then cooled to 20±5° C. and stirred at 20±5° C. for 16 hours. The white precipitates were collected by filtration and the cake was washed with heptane (2×5.1 L) and dried on the filter under nitrogen with a pulling vacuum. The solid was further dried in a vacuum oven at 55-65° C. with nitrogen purge to provide compound 25 (3.11 kg, 92.2%) as white to off-white powder. For 25:
ADIPATE OF INCB 39110
1H NMR (400 MHz, (CD3)2SO) δ 12.16 (s, 1H), 12.05 (brs, 2H), 8.85 (s, 1H), 8.72 (s, 1H), 8.69 (d, J=4.7 Hz, 1H), 8.45 (s, 1H), 7.93 (t, J=4.7 Hz, 1H), 7.63 (dd, J=3.6, 2.3 Hz, 1H), 7.09 (dd, J=3.6, 1.7 Hz, 1H), δ 4.11 (dt, J=11.0, 4.4 Hz, 1H), 3.77 (d, J=7.8 Hz, 2H), 3.60 (t, J=7.8 Hz, 2H), 3.58 (s, 2H), 3.44 (dt, J=14.4, 4.6 Hz, 1H), 3.28 (t, J=10.4 Hz, 1H), 3.09 (ddd, J=13.2, 9.6, 3.2 Hz, 1H), 2.58 (tt, J=8.6, 3.5 Hz, 1H), 2.28-2.17 (m, 4H), 1.83-1.74 (m, 1H), 1.67 (d, J=11.0 Hz, 1H), 1.59-1.46 (m, 4H), 1.37-1.21 (m, 2H) ppm;
13C NMR (101 MHz, (CD3)2SO) δ 174.38, 160.29, (153.52, 150.87), 152.20, 150.94, 149.63, (146.30, 146.25), 139.48, (134.79, 134.62), (135.08, 134.97, 134.74, 134.62, 134.38, 134.28, 134.04, 133.93), 129.21, 127.62, 126.84, 122.05, (124.75, 122.02, 119.29, 116.54), 117.39, 113.01, 99.99, 61.47, 60.50, 57.06, 44.24, 33.42, 30.70, 28.63, 27.89, 27.20, 24.07 ppm;
C32H33F4N9O5 (Mol. Wt: 699.66; free base: C26H23F4N9O (MW, 553.51), LCMS (EI) m/e 554.0 (M++H).
…………………………
WO-2014138168
http://www.google.com/patents/WO2014138168A1?cl=en
Processes for preparing JAK inhibitor (preferably INCB-39110) comprising the reaction of a substituted 1H-pyrazole compound with 4-chloro-7H-pyrrolo[2,3-d]pyrimidine in the presence of a base (eg cesium fluoride) and a solvent under Suzuki coupling conditions ([1,1'- bis(dicyclohexylphosphino)ferrocene]dichloropalladium (II)), followed by deprotection and then reaction with a piperidine derivative, and salt synthesis are claimed. Also claimed are novel intermediates and processes for their preparation. The compound is disclosed to be useful for treating disease mediated by JAK activity (targeting JAK-1 and 2), such as multiple sclerosis, rheumatoid arthritis, type I diabetes, inflammatory bowel disease, Crohn’s disease, COPD, prostate cancer, hepatic cancer, breast cancer, influenza, and SARS.
Example 1. Synthesis of 2-(3-(4-(7H-Pyrrolo[2,3-< ]pyrimidin-4-yl)-lH-pyrazol-l- yl)-l-(l-(3-fluoro-2-(trifluoromethyl)isonicotinoyl)piperidin-4-yl)azetidin-3- yl)acetonitrile Adipate (9)20443-0253WO1 (INCY0124-WO1) PATENT
tert-Butyl 3-(cyanomethyl)-3-(4-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)-lH- pyrazol-l-yl)azetidine-l-carboxylate (3). To a 1-L flask equipped with a nitrogen inlet, a thermocouple, and a mechanical stirrer were sequentially added isopropanol (IP A, 200 mL), l,8-diazabicyclo[5,4,0]undec-ene (DBU, 9.8 g, 64.4 mmol, 0.125 equiv), 4-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)-lH-pyrazole (1, 101 g, 520.51 mmol, 1.01 equiv) and tert-butyl 3-(cyanomethylene)azetidine-l-carboxylate (2, 100 g, 514.85 mmol) at ambient temperature to generate a reaction mixture as a
suspension. The resulting reaction mixture was heated to reflux in 30 minutes to provide a homogenous solution and the mixture was maintained at reflux for an additional 2 – 3 hours. After the reaction was complete as monitored by HPLC, n- heptane (400 mL) was gradually added to the reaction mixture in 45 minutes while maintaining the mixture at reflux. Solids were precipitated out during the w-heptane addition. Once w-heptane addition was complete, the mixture was gradually cooled to ambient temperature and stirred at ambient temperature for an additional 1 hour. The solids were collected by filtration, washed with w-heptane (200 mL), and dried under vacuum at 50 °C with nitrogen sweeping to constant weight to afford tert-butyl 3- 20443-0253WO1 (INCY0124-WO1) PATENT
(cyanomethyl)-3-(4-(4,4,5,5-tetramethyl- 1 ,3,2-dioxaborolan-2-yl)- IH-pyrazol- 1 – yl)azetidine-l -carboxylate (3, 181 g, 199.9 g theoretical, 90.5%) as a white to pale yellow solid. For 3: XH NMR (400 MHz, DMSO-i¾) δ 8.31 (s, 1H), 7.74 (s, 1H), 4.45 – 4.23 (m, 2H), 4.23 – 4.03 (m, 2H), 3.56 (s, 2H), 1.38 (s, 9H), 1.25 (s, 12H) ppm; 13C NMR (101 MHz, DMSO-i/6) δ 155.34, 145.50, 135.88, 1 16.88, 107.08 (br), 83.15, 79.36, 58.74 (br), 56.28, 27.96, 26.59, 24.63 ppm; Ci9H29B 404 (MW 388.27),
LCMS (EI) mle 389 (M+ + H). teri-Butyl 3-(4-(7H-pyrrolo[2,3-< |pyrimidin-4-yl)-lH-pyrazol-l-yl)-3- (cyanomethyl)-azetidine-l-carboxylate (5). To a 1-L flask equipped with a nitrogen inlet, a thermocouple, and a mechanical stirrer were added 4-chloro-7H-pyrrolo[2,3- i/]pyrimidine (4, 39.6 g, 257.6 mmol), tert-butyl 3-(cyanomethyl)-3-(4-(4,4,5,5- tetramethyl- 1 ,3 ,2-dioxaborolan-2-yl)- IH-pyrazol- 1 -yl)azetidine- 1 -carboxylate (3, 100 g, 257.6 mmol, 1.0 equiv), cesium fluoride (136.9 g, 901.4 mmol, 3.5 equiv), tert- butanol (250 mL), water (250 mL), and [l, l'-bis(di- cyclohexylphosphino)ferrocene]dichloropalladium(II) (Pd-127, 351.4 mg, 0.46 mmol, 0.0018 equiv) at ambient temperature. The resulting reaction mixture was de-gassed and refilled with nitrogen for 3 times before being heated to reflux and maintained at reflux under nitrogen for 20 – 24 hours. When HPLC showed the reaction was complete, the reaction mixture was cooled to 45 – 55 °C in 30 minutes, the two phases were separated, and the aqueous phase was discarded. To the organic phase was added w-heptane (125 mL) in 30 minutes at 45 – 55 °C. The resulting mixture was slowly cooled to ambient temperature in one hour and stirred at ambient temperature for an additional 2 hours. The solids were collected by filtration, washed with n- heptane (100 mL), and dried under vacuum at 50 °C with nitrogen sweeping to constant weight to afford tert-butyl 3-(4-(7H-pyrrolo[2,3-<i]pyrimidin-4-yl)-lH- pyrazol-l-yl)-3-(cyanomethyl)-azetidine-l -carboxylate (5, 96.8 g, 97.7 g theoretical, 99%) as a pale yellow solid. For 5: XH NMR (400 MHz, DMSO-i¾) δ 8.89 (s, 1H), 8.68 (s, 1H), 8.44 (s, 1H), 7.60 (d, J= 3.5 Hz, 1H), 7.06 (d, J= 3.6 Hz, 1H), 4.62 – 4.41 (m, 2H), 4.31 – 4.12 (m, 2H), 3.67 (s, 2H), 1.39 (s, 9H) ppm; 13C NMR (101 MHz, DMSO-i¾) δ 155.40, 152.60, 150.63, 149.15, 139.76, 129.53, 127.65, 122.25, 20443-0253WO1 (INCY0124-WO1) PATENT
116.92, 113.21, 99.71, 79.45, 58.34 (br), 56.80, 27.99, 26.83 ppm; Ci9H21 702 (MW 379.4), LCMS (EI) mle 380 (M+ + H).
2- (3-(4-(7H-Pyrrolo[2,3-< |pyrimidin-4-yl)-lH-pyrazol-l-yl)azetidin-3- yl)acetonitrile dihydrochloride salt (6). To a 0.5-L flask equipped with a nitrogen inlet, a thermocouple, an additional funnel, and a mechanical stirrer were added tert- butyl 3 -(4-(7H-pyrrolo [2,3 -<i]pyrimidin-4-yl)- lH-pyrazol- 1 -yl)-3 – (cyanomethyl)azetidine-l-carboxylate (5, 15 g, 39.5 mmol), water (7.5 mL, 416 mmol) and dichloromethane (75 mL) at room temperature. The mixture was stirred at room temperature to generate a suspension. To the suspension was added a solution of 5 M hydrogen chloride (HQ) in isopropanol (55 mL, 275 mmol, 7.0 equiv) in 5 minutes. The resulting reaction mixture was then heated to gentle reflux and
maitained at reflux for 3-4 hours. After the reaction was completed as mornitored by HPLC, tert-butyl methyl ether (TBME, 45 mL) was added to the reaction suspension. The mixture was gradually cooled to room temperature, and stirred for an additional one hour. The solids were collected by filtration, washed with tert-butyl methyl ether (TBME, 45 mL) and dried under vacuum at 50 °C with nitrogen sweeping to constant weight to afford 2-(3-(4-(7H-pyrrolo[2,3-i/]pyrimidin-4-yl)-lH-pyrazol-l-yl)azetidin-
3- yl)acetonitrile dihydrochloride salt (6, 13.6 g, 13.9 g theoretical, 98%) as an off- white to light yellow solid. For 6: XH NMR (400 MHz, D20) δ 8.96 (s, 1H), 8.81 (s, 1H), 8.49 (s, 1H), 7.78 (d, J= 3.8 Hz, 1H), 7.09 (d, J= 3.7 Hz, 1H), 4.93 (d, J= 12.8 Hz, 2H), 4.74 (d, J= 12.5 Hz, 2H), 3.74 (s, 2H) ppm; 13C NMR (101 MHz, D20) δ 151.35, 143.75, 143.33, 141.33, 132.03, 131.97, 115.90, 114.54, 113.85, 103.18, 59.72, 54.45 (2C), 27.02 ppm; Ci4H15Cl2N7 (Ci4H13N7 for free base, MW 279.30), LCMS (EI) mle 280 (M+ + H).
2-(3-(4-(7H-Pyrrolo[2,3-< |pyrimidin-4-yl)-lH-pyrazol-l-yl)-l-(l-(3-fluoro-2- (trifluoromethyl)isonicotinoyl)piperidin-4-yl)azetidin-3-yl)acetonitrile (8, Free Base). To a 0.5-L flask equipped with a nitrogen inlet, a thermocouple, an additional funnel, and a mechanical stirrer were added 2-(3-(4-(7H-pyrrolo[2,3-<i]pyrimidin-4- yl)-lH-pyrazol-l-yl)azetidin-3-yl)acetonitrile dihydrochloride salt (6, 20 g, 56.78 mmol), dichloromethane (200 mL) and triethylamine (TEA, 16.62 mL, 119.2 mmol, 20443-0253WO1 (INCY0124-WO1) PATENT
2.1 equiv) at ambient temperature. The mixture was stired at ambient temperature for 30 minutes before l-(3-fluoro-2-(trifluoromethyl)-isonicotinoyl)piperidin-4-one (7, 17.15 g, 57.91 mmol, 1.02 equiv) was added to the mixture. The mixture was then treated with sodium triacetoxyborohydride (25.34 g, 1 13.6 mmol, 2.0 equiv) in 5 minutes at ambient temperature (below 26 °C). The resulting reaction mixture was stirred at ambient temperature for 2 hours. After the reaction was complete as mornitored by HPLC, the reaction mixture was quenched with saturated aHC03 aqueous solution (200 mL). The two phases were separated and the aqueous phase was extracted with methylene chloride (200 mL). The combined organic phase was washed with 4% brine (100 mL) followed by solvent switch of methylene chloride to acetone by distillation. The resulting solution of the desired crude product (8) in acetone was directly used for the subsequent adipate salt formation. A small portion of solution was purified by column chromatography (S1O2, 0 – 10% of MeOH in EtOAc gradient elution) to afford the analytically pure 2-(3-(4-(7H-pyrrolo[2,3- i/]pyrimidin-4-yl)- lH-pyrazol- 1 -yl)- 1 -( 1 -(3 -fluoro-2-
(trifluoromethyl)isonicotinoyl)piperidin-4-yl)azetidin-3-yl)acetonitrile (8 free base) as an off-white solid. For 8: ¾ NMR (400 MHz, DMSO-i¾) δ 12.17 (d, J= 2.8 Hz, 1H), 8.85 (s, 1H), 8.70 (m, 2H), 8.45 (s, 1H), 7.93 (t, J= A J Hz, 1H), 7.63 (dd, J= 3.6, 2.3 Hz, 1H), 7.09 (dd, J= 3.6, 1.7 Hz, 1H), 4.10 (m, 1H), 3.78 (d, J= 7.9 Hz, 2H), 3.61 (t, J= 7.9 Hz, 1H), 3.58 (s, 2H), 3.46 (m, 1H), 3.28 (t, J= 10.5 Hz, 1H), 3.09 (ddd, J = 13.2, 9.5, 3.1 Hz, 1H), 2.58 (m, 1H), 1.83 – 1.75 (m, 1H), 1.70 – 1.63 (m, 1H), 1.35 – 1.21 (m, 2H) ppm; 13C MR (101 MHz, DMSO-i/6) δ 160.28, (153.51, 150.86), 152.20, 150.94, 149.62, (146.30, 146.25), 139.48, (134.78, 134.61), (135.04, 134.92, 134.72, 134.60, 134.38, 134.26, 134.03, 133.92), 129.22, 127.62, 126.84, 121.99, 122.04, (124.77, 122.02, 1 19.19, 1 16.52), 117.39, 113.00, 99.99, 61.47, 60.49, 57.05, 44.23, 28.62, 27.88, 27.19 ppm;
(MW, 553.51), LCMS (EI) mle 554.1 (M+ + H).
2-(3-(4-(7H-Pyrrolo[2,3-< |pyrimidin-4-yl)-lH-pyrazol-l-yl)-l-(l-(3-fluoro-2- (trifluoromethyl)isonicotinoyl)piperidin-4-yl)azetidin-3-yl)acetonitrile Adipate (9). To a 0.5-L flask equipped with a mechanical stirrer, a thermocouple, an addition funnel, and a nitrogen inlet was added a solution of crude 2-(3-(4-(7H-pyrrolo[2,3- 20443-0253WO1 (INCY0124-WO1) PATENT i/]pyrimidin-4-yl)- lH-pyrazol- 1 -yl)- 1 -( 1 -(3 -fluoro-2-
(trifluoromethyl)isonicotinoyl)piperidin-4-yl)azetidin-3-yl)acetonitrile (8 free base, 31.38 g, 56.7 mmol) in acetone (220 mL) and adipic acid (8.7 g, 59.53 mmol, 1.05 equiv) at ambient temperature. The reaction mixture was then heated to reflux to give a solution. w-Heptane (220 mL) was gradually added to the reaction mixture at 40 – 50 °C in one hour. The resulting mixture was gradually cooled to ambient temperature in one hour and stirred at ambient temperature for an additional 16 hours. The solids were collected by filtration, washed with w-heptane (2 X 60 mL), and dried under vacuum at 50 °C with nitrogen sweeping to constant weight to afford 2-(3-(4-(7H- Pyrrolo[2,3 -i/]pyrimidin-4-yl)- lH-pyrazol- 1 -yl)- 1 -(1 -(3 -fluoro-2- (trifluoromethyl)isonicotinoyl)piperidin-4-yl)azetidin-3-yl)acetonitrile adipate (9,34.0 g, 39.7 g theoretical, 85.6% for two steps) as a white to off-white solid. 9:
XH NMR (400 MHz, DMSO-i/6) δ 12.16 (s, 1H), 12.05 (brs, 2H), 8.85 (s, 1H), 8.72 (s, 1H), 8.69 (d, J= A J Hz, 1H), 8.45 (s, 1H), 7.93 (t, J= A J Hz, 1H), 7.63 (dd, J= 3.6, 2.3 Hz, 1H), 7.09 (dd, J= 3.6, 1.7 Hz, 1H), 5 4.1 1 (dt, J= 1 1.0, 4.4 Hz, 1H), 3.77 (d, J= 7.8 Hz, 2H), 3.60 (t, J= 7.8 Hz, 2H), 3.58 (s, 2H), 3.44 (dt, J= 14.4, 4.6 Hz, 1H), 3.28 (t, J= 10.4 Hz, 1H), 3.09 (ddd, J= 13.2, 9.6, 3.2 Hz, 1H), 2.58 (tt, J= 8.6, 3.5 Hz, lH), 2.28 – 2.17 (m, 4H), 1.83 – 1.74 (m, 1H), 1.67 (d, J= 11.0 Hz, 1H), 1.59 – 1.46 (m, 4H), 1.37 – 1.21 (m, 2H) ppm;
13C MR (101 MHz, DMSO-i/6) δ 174.38, 160.29, (153.52, 150.87), 152.20, 150.94, 149.63, (146.30, 146.25), 139.48, (134.79, 134.62), (135.08, 134.97, 134.74, 134.62, 134.38, 134.28, 134.04, 133.93), 129.21, 127.62, 126.84, 122.05, (124.75, 122.02, 1 19.29, 1 16.54), 117.39, 113.01, 99.99, 61.47, 60.50, 57.06, 44.24, 33.42, 30.70, 28.63, 27.89, 27.20, 24.07 ppm;
C32H33F4N9O5 ( MW 699.66;![Figure imgf000043_0001]() for free base, MW, 553.51), LCMS (EI) mle 554.0 (M+ + H).
for free base, MW, 553.51), LCMS (EI) mle 554.0 (M+ + H).
Example 2: Alternative Synthesis of 2-(3-(4-(7H-Pyrrolo[2,3-d]pyrimidin-4-yl)- lH-pyrazol-l-yl)-l-(l-(3-fluoro-2-(trifluoromethyl)isonicotinoyl)piperidin-4- yl)azetidin-3-yl)acetonitrile 20443-0253WO1 (INCY0124-WO1) PATENT
Scheme II
………………………………..COMPD11……………………………………………………………………………………………………..COMPD 8 BASE
C26H3i BF4N603 C26H23F4N9O Mol. Wt: 562.37 Mol. Wt: 553.51
2- (Azetidin-3-ylidene)acetonitrile hydrochloride (2a). To a 0.5-L flask equipped with a nitrogen inlet, a thermocouple, and a mechanical stirrer were added tert-butyl
3- (cyanomethylene)azetidine-l-carboxylate (2, 30 g, 154.46 mmol) and
methylenechloride (300 mL) at ambient temperature. The solution was then treated with a solution of 5 M hydrogen chloride (HQ) in isopropanol solution (294.2 mL, 1.54 mol, 10 equiv) at ambient temperature and the resulting reaction mixture was stirred at ambient temperature for 18 hours. After the reaction was complete as monitored by HPLC, the suspension was added tert-butyl methyl ether (TBME, 150 mL), and the mixture was stirred at ambient temperature for 2 hours. The solids was collected by filtration, washed with w-heptane (2 X 100 mL), and dried on the filtration funnel at ambient temperature for 3 hours to afford 2-(azetidin-3- ylidene)acetonitrile hydrochloride (2a, 13.7 g, 20.2 g theoretical, 67.8 %) as a white solid. For 2a: XH NMR (500 MHz, DMSO-i¾) δ 9.99 (s, 2H), 5.94 (p, J= 2.5 Hz, 1H), 20443-0253WO1 (INCY0124-WO1) PATENT
4.85 – 4.80 (m, 2H), 4.77 – 4.71 (m, 2H) ppm; C NMR (126 MHz, DMSO-i¾) δ 155.65, 114.54, 94.78, 55.26, 54.63 ppm; C5H7C1N2 (MW 130.58; C5H6N2 for free base, MW 94.11), LCMS (EI) mle 95 (M+ + H).
2-(l-(l-(3-Fluoro-2-(trifluoromethyl)isonicotinoyl)piperidin-4-yl)azetidin-3- ylidene)acetonitrile (10). To a 0.25-L flask equipped with a nitrogen inlet, a thermocouple, and a magnetic stirrer were added 2-(azetidin-3-ylidene)acetonitrile hydrochloride (2a, 4.5 g, 34.46 mmol), l-(3-fluoro-2-
(trifluoromethyl)isonicotinoyl)piperidin-4-one (7, 10 g, 34.46 mmol, 1.0 equiv), and methylenechloride (100 mL) at ambient temperqature and the resulting mixture was then treated with sodium triacetoxyborohydride (14.6 g, 68.93 mmol, 2.0 equiv) at ambient temperature. The reaction mixture was stirred at ambient temperature for 2 hours before being quenched with saturated sodium bicarbonate (NaHCOs) aqueous solution (50 mL). The two phases were separated and the aqueous phase was extracted with dichloromethane (200 mL). The combined organic phase was washed with water (50 mL) and brine (50 mL) and concentrated under reduced pressure to afford the crude desired product (10), which was purified by column chromatography (S1O2, 0 – 10 % of ethyl acetate in hexane gradient elution) to afford 2-(l-(l-(3- fluoro-2-(trifluoromethyl)isonicotinoyl)piperidin-4-yl)azetidin-3-ylidene)acetonitrile (10, 9.5 g, 12.7 g theoretical, 74.8 %) as a white solid. For 10: XH NMR (400 MHz, CDCI3) δ 8.57 (d, J= A J Hz, 1H), 7.54 (t, J= 4.6 Hz, 1H), 5.29 (p, J= 2.4 Hz, 1H), 4.18 – 4.08 (m, 1H), 4.08 – 4.03 (m, 2H), 3.98 – 3.94 (m, 2H), 3.57 – 3.39 (m, 2H), 3.17 – 3.04 (m, 1H), 2.56 (tt, J= 7.4, 3.5 Hz, 1H), 1.86 – 1.77 (m, 1H), 1.75 – 1.64 (m, 1H), 1.54 – 1.43 (m, 1H), 1.43 – 1.31 (m, lH) ppm; 13C MR (101 MHz, CDC13) δ 161.34, 160.73, 152.62 (d, J= 269.1 Hz), 145.75 (d, J= 6.1 Hz), 136.73 (qd, J = 36.1, 12.0 Hz), 134.56 (d, J= 16.9 Hz), 126.89, 120.58 (qd, J= 275.0, 4.9 Hz),
115.11, 92.04, 62.05, 60.57 (2C), 44.47, 39.42, 29.38, 28.47 ppm; Ci7H16F4N40 (MW 368.33), LCMS (EI) mle 369 (M++ H).
2-(l-(l-(3-Fluoro-2-(trifluoromethyl)isonicotinoyl)piperidin-4-yl)-3-(4-(4,4,5,5- tetramethyl-l,3,2-dioxaborolan-2-yl)-lH-pyrazol-l-yl)azetidin-3-yl)acetonitrile (11). To a 25 mL flask equipped with a nitrogen inlet, a thermocouple, and a magnetic 20443-0253WO1 (INCY0124-WO1) PATENT stirrer were added 4-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)-lH-pyrazole (1, 210 mg, 1.08 mmol, 1.08 equiv), 2-(l-(l-(3-fluoro-2-
(trifluoromethyl)isonicotinoyl)piperidin-4-yl)azetidin-3 -ylidene)acetonitrile (10, 370 mg, 1.0 mmol) and acetonitrile (3 mL) at ambient temperature. The solution was then treated with l,8-diazabicyclo[5,4,0]undec-ene (DBU, 173 mg, 0.17 mL, 1.12 mmol, 1.12 equiv) at ambient temperature and the resulting reaction mixture was warmed to 50 °C and stirred at 50 °C for overnight. When the reaction was complete as
monitored by HPLC, the reaction mixture was directly load on a solica gel (S1O2) column for chromatographic purification (0 – 2.5 % MeOH in ethyl acetate gradient elution) to afford 2-(l-(l-(3-fluoro-2-(trifluoromethyl)isonicotinoyl)piperidin-4-yl)-3- (4-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)-lH-pyrazol-l-yl)azetidin-3- yl)acetonitrile
![Figure imgf000010_0003]() COMPD 11
COMPD 11
(11, 263 mg, 562.4 mg theoretical, 46.7 %) as a white solid.
For 11: ΧΗ NMR (400 MHz, DMSO-i/6) δ 8.64 (d, J= 4.7 Hz, 1H), 8.22 (d, J= 0.6 Hz, 1H), 7.88 (dd, J= A J Hz, 1H), 7.69 (s, 1H), 4.10 – 3.99 (m, 1H), 3.58 (d, J= 7.8 Hz, 2H), 3.52 – 3.42 (m, 2H), 3.44 (s, 2H), 3.41 – 3.33 (m, 1H), 3.28 – 3.15 (m, 1H), 3.03 (ddd, J= 12.9, 9.2, 3.2 Hz, 1H), 2.51 – 2.44 (m, 1H), 1.77 – 1.66 (m, 1H), 1.64 – 1.54 (m, 1H), 1.28 – 1.17 (m, 2H), 1.24 (s, 12H) ppm;
13C MR (101 MHz, DMSO-i/6) δ 160.22, 152.13 (d, J= 265.8 Hz), 146.23 (d, J= 5.7 Hz), 145.12, 135.41, 134.66 (d, J= 16.9 Hz), 134.43 (qd, J= 35.0, 1 1.7 Hz), 127.58, 120.61 (qd, J= 274.4, 4.6 Hz), 117.35, 106.59 (br), 83.10, 61.40, 60.53 (2C), 56.49, 44.17, 38.99, 28.55, 27.82, 27.02, 24.63 ppm; C26H3iBF4 603 (MW 562.37), LCMS (EI) mle 563 (M+ + H).
2-(3-(4-(7H-Pyrrolo[2,3-< |pyrimidin-4-yl)-lH-pyrazol-l-yl)-l-(l-(3-fluoro-2- (trifluoromethyl)isonicotinoyl)piperidin-4-yl)azetidin-3-yl)acetonitrile (8). To a
25-mL flask equipped with a nitrogen inlet, a thermocouple, an additional funnel, and a magnetic stirrer were added 2-(l-(l-(3-fluoro-2-(trifluoromethyl)- isonicotinoyl)piperidin-4-yl)-3-(4-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)-lH- pyrazol-l-yl)azetidin-3-yl)acetonitrile (11, 307 mg, 0.546 mmol), 4-chloro-7H- pyrrolo[2,3-if|pyrimidine (4, 84.8 mg, 0.548 mmol, 1.0 equiv), sodium bicarbonate (NaHC03, 229 mg, 2.72 mmol, 5.0 equiv), water (1.6 mL), and 1,4-dioxane (1.6 mL) at ambient temperature. The mixture was then teated with
tetrakis(triphenylphosphine)palladium(0) (12.8 mg, 0.011 mmol, 0.02 equiv) at 20443-0253WO1 (INCY0124-WO1) PATENT ambient temperature and the resulting reaction mixture was de-gassed and refilled with nitrogen for 3 times before being heated to 85 °C. The reaction mixture was stired at 85 °C under nitrogen for overnight. When the reaction was complete as monitored by HPLC, the reaction mixture was concentrated to dryness under reduced pressure and the desired product, 2-(3-(4-(7H-pyrrolo[2,3-( Jpyrimidin-4-yl)-lH- pyrazol- 1 -yl)- 1 -( 1 -(3 -fluoro-2-(trifluoromethyl)isonicotinoyl)piperidin-4-yl)azetidin- 3-yl)acetonitrile (8 free base, 135 mg, 302.2 mg theoretical, 44.6 %), was obtained as off- white solids by direct silica gel (S1O2) cloumn chromatography (0 – 10% of ethyl acetate in hexane gradient elution) purification of the dried reaction mixture. The compound obtained by this synthetic approach is identical in every comparable aspect to the compound 8 manufactured by the synthetic method as described above inExample 1.
……………………………………………….
A Double-Blind, Placebo-Controlled Study Exploring the Safety, Tolerability, and Efficacy of a 28 Day Course of INCB-039110 in Subjects With Active Rheumatoid Arthritis (NCT01626573)
ClinicalTrials.gov Web Site 2012, June 25
A double-blind, placebo-controlled study exploring the safety, tolerability, and efficacy of a 28-day course of escalating doses of an oral JAK 1 inhibitor (INCB039110) in subjects with stable, chronic plaque psoriasis
22nd Congr Eur Acad Dermatol Venereol (EADV) (October 3-6, Istanbul) 2013, Abst FC01.6
A randomized, dose-ranging, placebo-controlled, 84-day study of INCB039110, a selective janus kinase-1 inhibitor, in patients with active rheumatoid arthritis
77th Annu Sci Meet Am Coll Rheumatol (October 26-30, San Diego) 2013, Abst 1797
Safety Study of INCB-039110 in Combination With Gemcitabine and Nab-Paclitaxel in Subjects With Advanced Solid Tumors (NCT01858883)
ClinicalTrials.gov Web Site 2013, May
An Open-Label, Phase II Study Of The JAK1 Inhibitor INCB039110 In Patients With Myelofibrosis
55th Annu Meet Am Soc Hematol (December 7-10, New Orleans) 2013, Abst 663
| WO2013036611A1 * |
Sep 6, 2012 |
Mar 14, 2013 |
Incyte Corporation |
Processes and intermediates for making a jak inhibitor |
| WO2013043962A1 * |
Sep 21, 2012 |
Mar 28, 2013 |
Merck Sharp & Dohme Corp. |
Cyanomethylpyrazole carboxamides as janus kinase inhibitors |
Filed under:
cancer,
Phase2 drugs Tagged:
CANCER,
INCB-039110,
INCB-39110,
INCYTE,
JAK-1 inhibitor,
Janus kinase-1 (JAK-1) inhibitor,
myelofibrosis,
phase 2,
plaque psoriasis,
rheumatoid arthritis ![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
















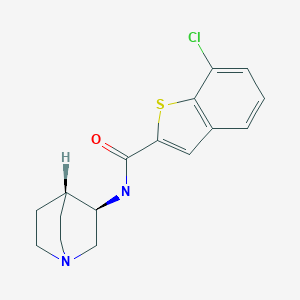
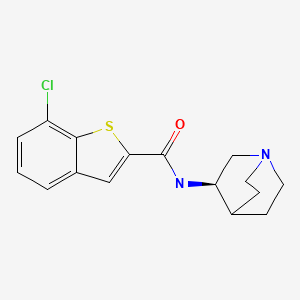







![[1860-5397-7-57-i76]](http://www.beilstein-journals.org/bjoc/content/inline/1860-5397-7-57-i76.png?max-width=550&background=EEEEEE)


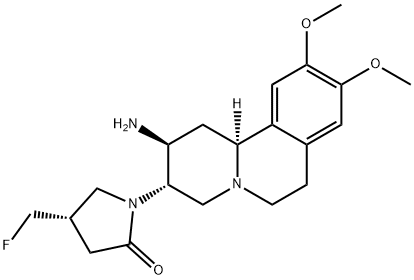

![[1860-5397-9-265-7]](http://www.beilstein-journals.org/bjoc/content/figures/1860-5397-9-265-7.png?max-width=550&background=EEEEEE)
![[1860-5397-9-265-i29]](http://www.beilstein-journals.org/bjoc/content/inline/1860-5397-9-265-i29.png?max-width=550&background=EEEEEE)
![[1860-5397-9-265-8]](http://www.beilstein-journals.org/bjoc/content/figures/1860-5397-9-265-8.png?max-width=550&background=EEEEEE)
![[1860-5397-9-265-i30]](http://www.beilstein-journals.org/bjoc/content/inline/1860-5397-9-265-i30.png?max-width=550&background=EEEEEE)


























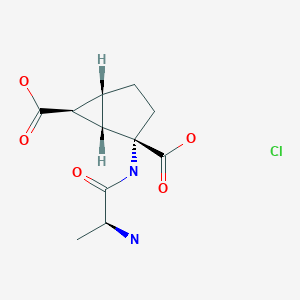
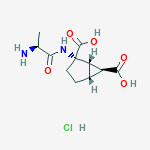














 SERTRALINE
SERTRALINE



















![[1860-5397-7-57-i86]](http://www.beilstein-journals.org/bjoc/content/inline/1860-5397-7-57-i86.png?max-width=550&background=EEEEEE)













 INCB-39110,
INCB-39110, ADIPATE OF INCB-39110
ADIPATE OF INCB-39110
























 COMPD 11
COMPD 11